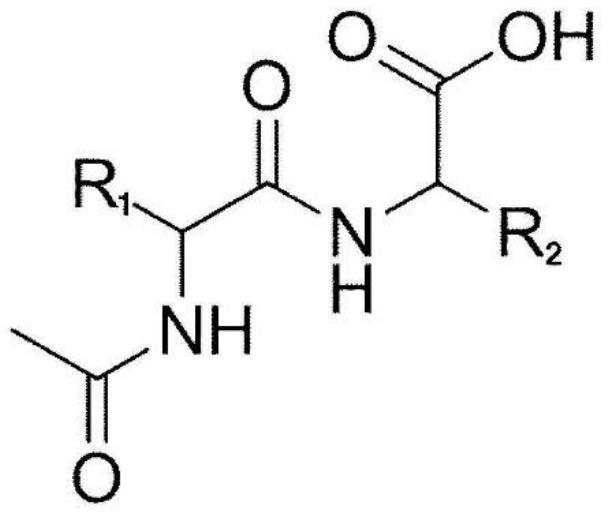
Method for producing n-acetyl dipeptide and n-acetyl amino acid
A technology for acetyl dipeptide and amino acid, applied in the field of preparing N-acetyl dipeptide and N-acetyl amino acid, can solve the problems of long manufacturing time, rising manufacturing cost, difficulty in dipeptide mixture and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0051] Example 1. Selectivity of N-acetylmethionine dipeptide (NALM-Met) according to catalyst type
[0052] In order to compare the selectivity of N-acetylmethionine dipeptide (hereinafter referred to as 'NALM-Met') and N-acetylmethionine (hereinafter referred to as 'NALM') according to the type of catalyst, methionine (0.67 mol), acetic anhydride (0.70 moles), and acetic acid (1.83 moles), to which 0.025 moles of one of the following items: calcium hydroxide (Ca(OH) 2 ), calcium chloride (CaCl 2 ), basic copper carbonate (Cu(OH) 2 CO 3 ), copper sulfate (CuSO 4 ), ammonium chloride (NH 4 Cl), sodium acetate (CH 3 CO 2 Na), phosphoric acid (H 3 PO 4 ), and sodium chloride (NaCl), and stirred at room temperature for 4 hours. An experimental group to which no salt was added under the same conditions was used as a control group. After confirming by using thin layer chromatography (thin layer chromatography; TLC), when the reaction is finished, a sample is taken and the...
Embodiment 2
[0058] Example 2. According to Ca(OH) 2 Selectivity of the amount of NALM-Met used
[0059] In order to compare the Ca(OH) 2 The selectivity of the amount of use is to mix methionine (0.67 mol), acetic anhydride (0.70 mol), and acetic acid (1.83 mol) and add 0%, 2.5%, 3.75%, 5%, and 10% to the methionine mole. , 20%, or 40% Ca(OH) 2 , and stirred at room temperature for 4 hours. Use does not use Ca(OH) 2 The experimental group served as the control group. After confirming by using thin-layer chromatography, take a sample when the reaction ends and perform high-performance liquid chromatography to confirm the concentration after the sample is diluted to 500 times, then, calculate the selectivity according to the calculation formula 1, and the results are shown in Table 2 middle.
[0060] [Table 2]
[0061]
[0062] According to the experimental results, it was confirmed that after adding Ca(OH) 2 The NALM-Met selectivity relative to the control group was improved in ...
Embodiment 3
[0063] Example 3. Selectivity of NALM-Met according to the amount of acetic acid used
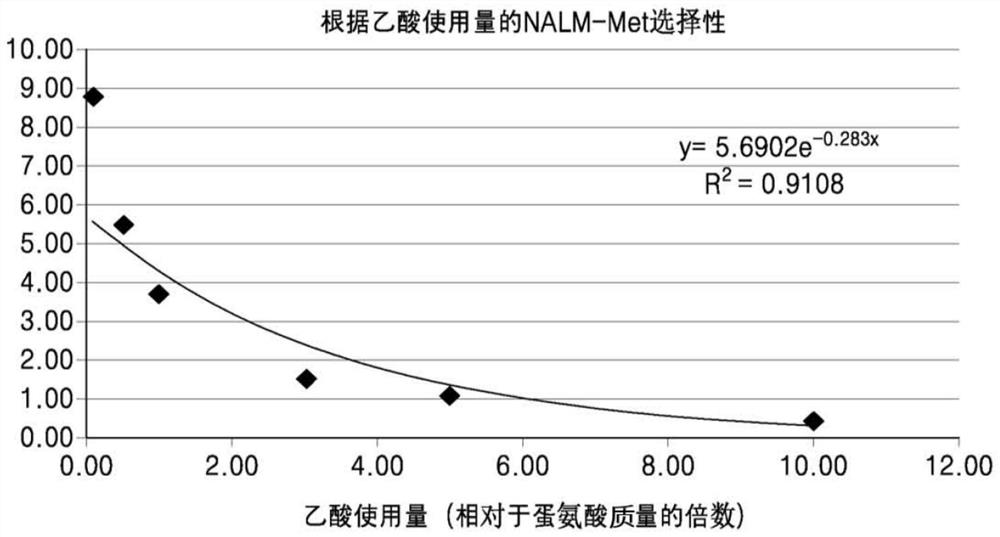
[0064] In order to compare the selectivity of N-acetylmethionine dipeptide according to the amount of acetic acid used as solvent, methionine (0.67 mol), acetic anhydride (0.70 mol), and Ca(OH) 2 (0.025 moles), and with respect to the mass of methionine 0.1 times, 0.5 times, 1.0 times, 3.0 times, 5.0 times, or 10.0 times the quality of acetic acid and stirred at room temperature for 4 hours. After confirming by using thin layer chromatography, take a sample when the reaction ends and carry out high performance liquid chromatography to confirm the concentration after the sample is diluted to 500 times, then, calculate the selectivity according to the calculation formula 1, and the results are shown in the table 3 and figure 1 middle.
[0065] [table 3]
[0066]
[0067] According to the experimental results, there is a significant exponential function relationship between the amount of ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
| boiling point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com