Crystal form of dydrogesterone intermediate and preparation method thereof
A kind of technology of dydrogesterone and intermediate, applied in the field of medicinal chemistry
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
[0050] The preparation of the crystal form A of example 1 drogesterone intermediate
[0051] Add 20g of dydrogesterone intermediate, 5ml of dichloromethane, and 50ml of acetone into the reaction flask in sequence, raise the temperature to 50-60°C, keep the temperature at reflux for 1 hour, and slowly cool down to 0-10°C to crystallize for 6-8 hours. After suction filtration, the filter cake was washed with 10 ml of cooled acetone, and the filter cake was air-dried at 50-60° C. to constant weight to obtain 18 g of off-white crystals with a HPLC purity of 99.80% and a yield of 90%.
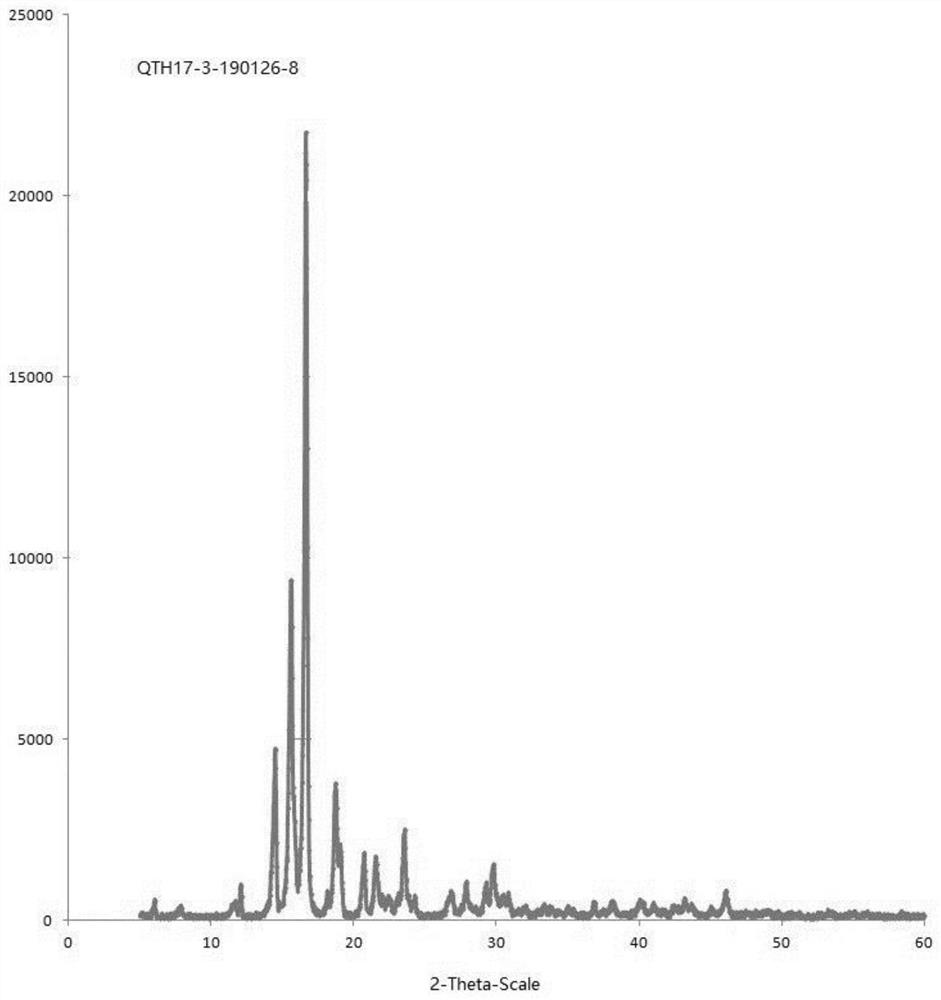
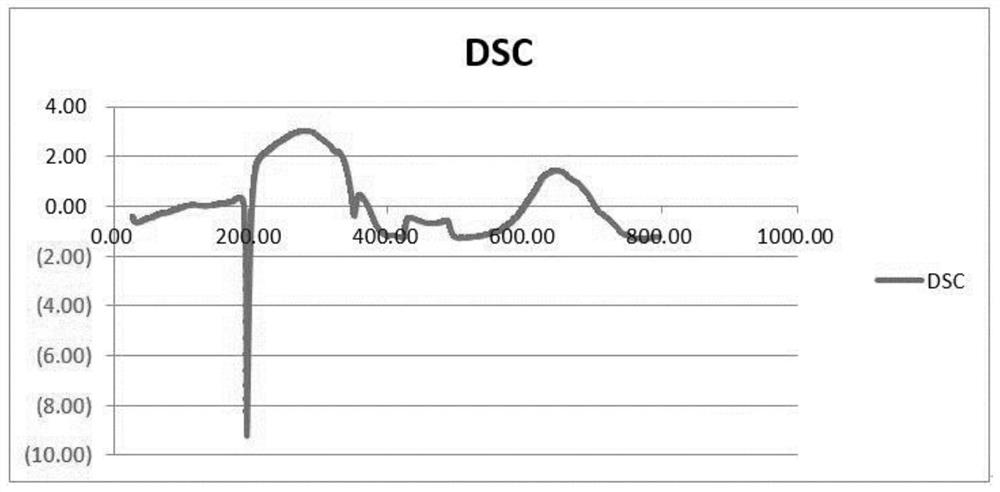
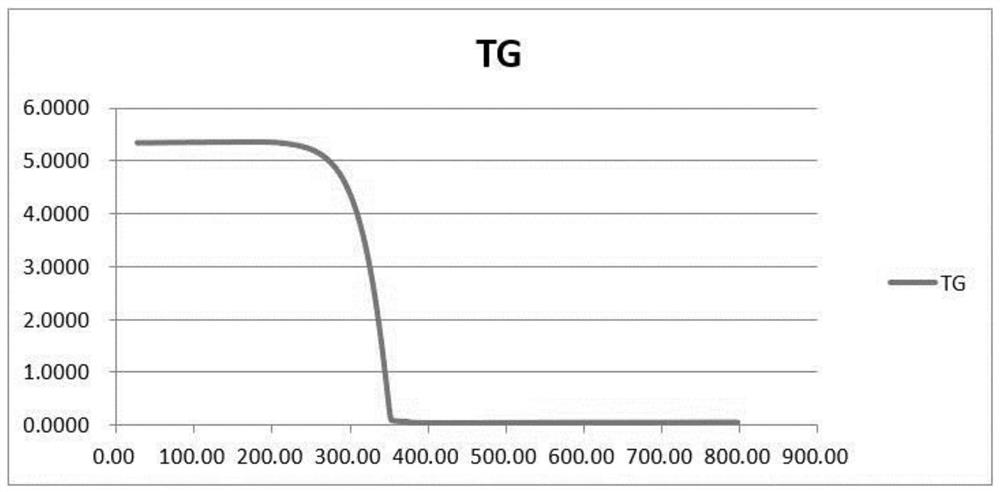
[0052] Gained crystal carries out X-ray powder diffraction (XRPD) analysis (see appendix figure 1 ), DSC analysis (see attached figure 2 ) and TGA analysis (attached image 3 ), is crystal form A.
example 2
[0053] The preparation of the crystal form A of example 2 drogesterone intermediate
[0054] Add 30g of dydrogesterone intermediate, 5ml of chloroform, and 50ml of acetone into the reaction flask in sequence, raise the temperature to 60-70°C, keep warm and reflux for 1 hour, and slowly cool down to 0-10°C to crystallize for 6-8 hours. After suction filtration, the filter cake was washed with 10 ml of cooled acetone, and the filter cake was air-dried at 50-60° C. to constant weight to obtain 17.2 g of off-white crystals with a HPLC purity of 99.85% and a yield of 86%. It was detected as crystal form A.
example 3
[0055]The preparation of the crystal form A of example 3 drogesterone intermediate
[0056] Add 30g of dydrogesterone intermediate, 5ml of tetrahydrofuran, and 70ml of acetone into the reaction flask in sequence, raise the temperature to 60-70°C, keep warm and reflux for 1 hour, and slowly cool down to 0-10°C to crystallize for 6-8 hours. After suction filtration, the filter cake was washed with 10 ml of cooled acetone, and the filter cake was air-dried at 50-60° C. to constant weight to obtain 18.2 g of off-white crystals with a HPLC purity of 99.91% and a yield of 91%. It was detected as crystal form A.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com