Crystal form of cyclohexane derivative
A technology of cyclohexane and derivatives, applied in the crystal form of cyclohexane derivatives and the field of preparation thereof, can solve the problems such as undisclosed crystal form of the compound of formula I, reduce production costs, facilitate long-term storage and transportation, The effect of low hygroscopicity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0045] Embodiment 1: N'-[trans-4-[2-[7-(benzo[b]thiophene)-7-piperazinyl]ethyl]cyclohexyl]-N,N-dimethylurea ( Formula I compound) preparation
[0046] Refer to the preparation of Example 5 of CN106518841A.
[0047] Preparation of 1-benzo[b]thiophene-4-piperazine hydrochloride
[0048]
[0049] 7.20g 7-bromobenzo[b]thiophene, 19.9g piperazine anhydride, 4.70g sodium tert-butoxide, 0.32g (R)-(+)-2,2′-bis(diphenylphosphino)- A mixture of 1,1'-binaphthalene (BINAP), 0.63 g of dipalladium tris(dibenzylideneacetone) and 150 ml of toluene was refluxed for 1 hour under a nitrogen atmosphere. Pour 150ml of water into the reaction solution, then extract with 100ml×3 ethyl acetate, wash with water, dry over anhydrous magnesium sulfate, and evaporate the solvent under reduced pressure (0.01MPa, 45°C). The residue was purified by silica gel column chromatography (dichloromethane:methanol:25% aqueous ammonia=100:10:1) to obtain 4.60 g of 1-benzo[b]thiophen-4-yl-piperazine as a yellow ...
Embodiment 2
[0059] Preparation and identification of embodiment 2 crystal form A
[0060] Dissolve 200 mg of the amorphous product from Example 1 in ethyl acetate, reflux at 77°C, cool to room temperature (20-25°C) and stir for 1 hour, filter with suction, and recrystallize to obtain a crystal form, which is named as the compound of formula I Form A.
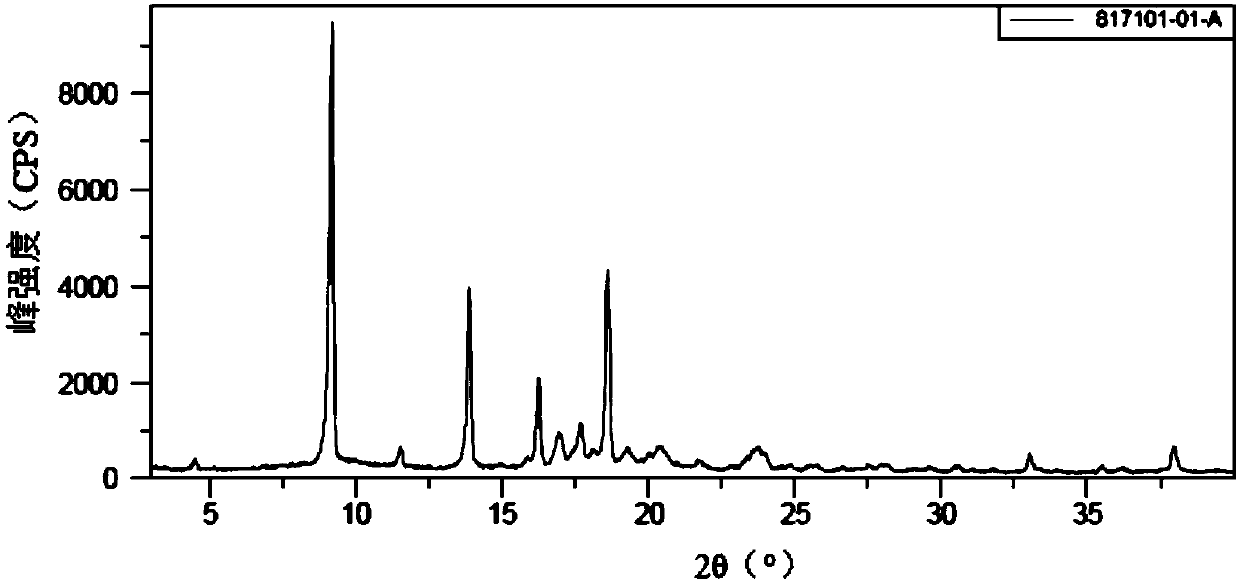
[0061] figure 1 The X-ray diffraction pattern (XRPD) of Form A is shown and the corresponding spacing values at 2θ are provided in Table 1 characteristic peaks.
[0062] Table 1
[0063]
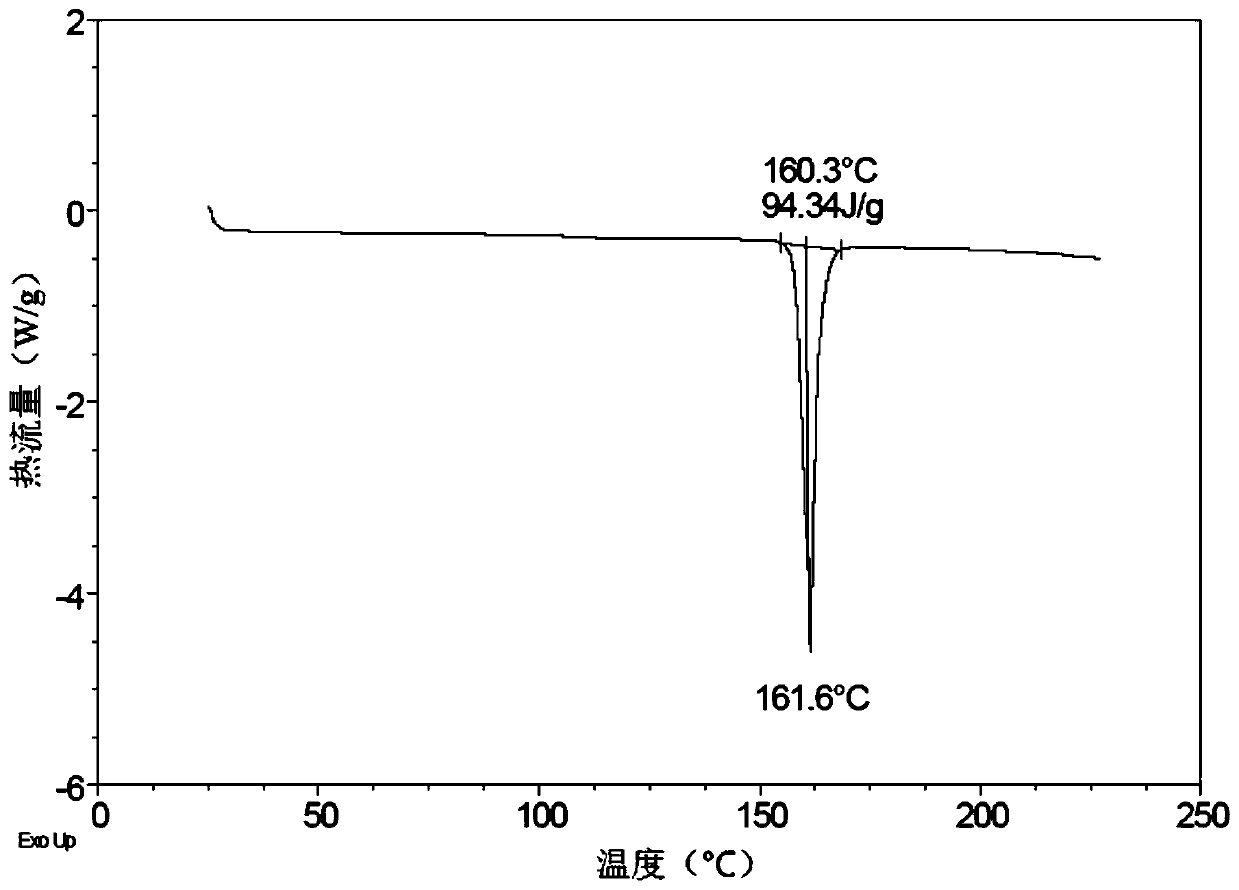
[0064] figure 2 The DSC chart of Form A is shown, showing an absorption peak, and the result shows that the sample has an endothermic peak at 161.6°C.
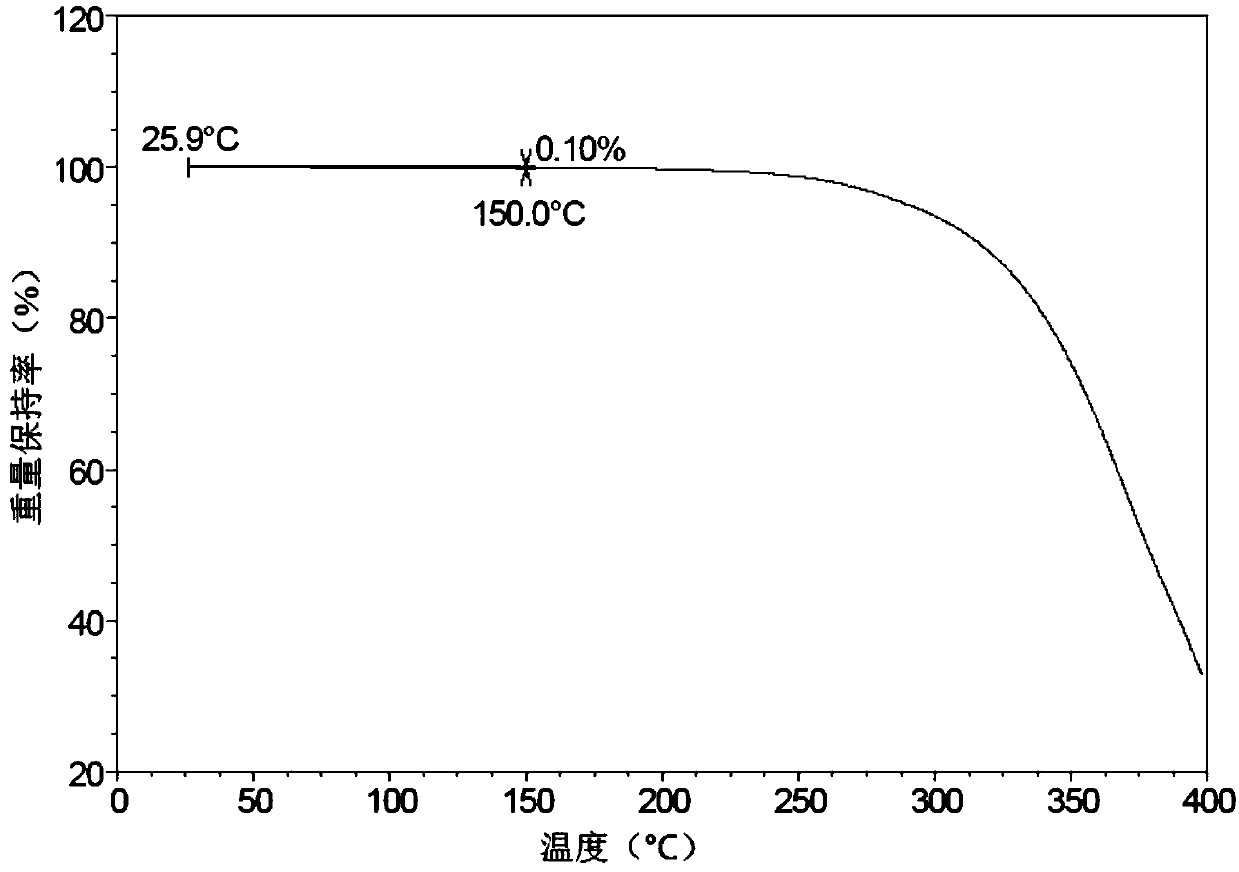
[0065] image 3 The TGA diagram of Form A is shown, and the TG shows that the sample has 0.1% weight loss when the sample is heated to 150°C.
Embodiment 3
[0066] Embodiment 3 Solubility experiment
[0067] Accurately weigh 20 mg of the crystal form A of Example 2 into a 4ml centrifuge tube, add 4ml of water, rotate and mix (25rpm) at 37°C for 1, 2, 4 and 24 hours, take samples at each time point and centrifuge, and measure Filtrate HPLC concentration and solubility, as shown in table 2 through HPLC measurement result:
[0068] Table 2
[0069] sample / time 1 hour 2 hours 4 hours 24 hours Example 2 Crystal Form A 0.028mg / ml 0.046mg / ml 0.031mg / ml 0.031mg / ml
[0070] Regarding Form A of the compound of formula I, the solubility in water is less than 0.1 mg / ml.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com