Cell membrane receptor protein identification method and verification method
A technology of receptor protein and membrane receptor, which is applied in the field of identification of cell membrane receptor protein, can solve the problems of low efficiency, heavy workload, and unsatisfactory identification effect of membrane receptor protein
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0044] Modification of rLZ-8 on the gel;
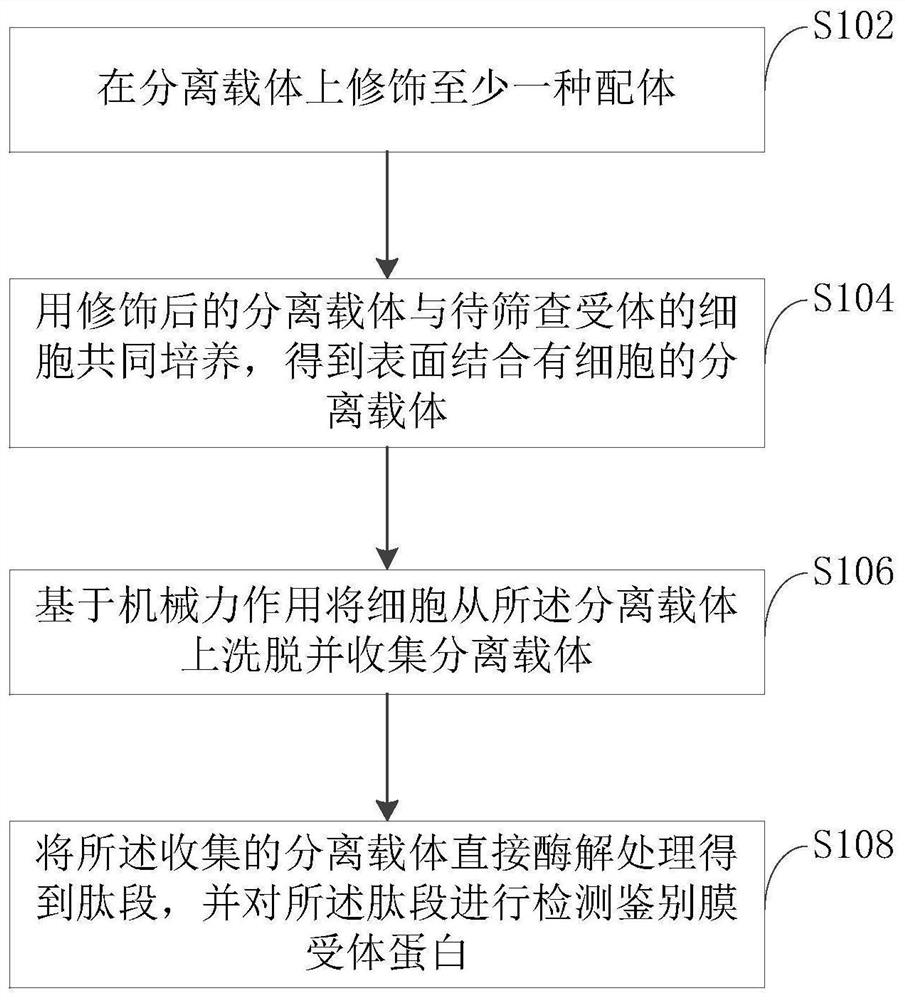
[0045] Co-cultivate the modified gel with lymphocytes for 1 hour to obtain a gel with lymphocytes bound to the surface;
[0046] Quickly pipette the cell-gel mixture 10 times with a blue pipette tip, then centrifuge at 200rpm for 2 minutes, discard the supernatant containing cells and collect the beads, and resuspend the gel with normal saline;
[0047] Repeat the previous step 2 times to obtain a gel in which cells are eluted and removed;
[0048] The gel beads were treated with enzymatic hydrolysis, and then the peptides were detected by UPLC-ESI-QTOF technology, and the protein was identified by searching the protein database, and it was determined that the receptor of rLZ-8 on the surface of lymphocytes was PSAP.
Embodiment 2
[0050] Modification of rLZ-8 on the gel;
[0051] The modified gel was co-cultured with lymphocytes for 2 hours to obtain a gel with lymphocytes bound to the surface;
[0052] Quickly pipette the cell-gel mixture 10 times with a blue pipette tip, then centrifuge at 400rpm for 1 minute, discard the supernatant containing cells and collect the beads, and resuspend the gel with normal saline;
[0053] Repeat the previous step 3 times to obtain a gel from which the cells are eluted;
[0054] The gel beads were treated with enzymatic hydrolysis, and then the peptide was detected by nano-UPLC-orbitrap technology, and the protein was identified by searching the protein database, and it was determined that the receptor of rLZ-8 on the surface of lymphocytes was PSAP.
Embodiment 3
[0056] Modification of rLZ-8 on the gel;
[0057] The modified gel was co-cultured with lymphocytes for 1.5 hours to obtain a gel with lymphocytes bound to the surface;
[0058] Quickly pipette the cell-gel mixture 10 times with a blue pipette tip, then centrifuge at 300rpm for 1.5 minutes, discard the supernatant containing cells and collect the beads, and resuspend the gel with physiological saline;
[0059] Repeat the previous step 3 times to obtain a gel from which the cells are eluted;
[0060] The gel beads were treated with enzymatic hydrolysis, and then the peptides were detected by MALDI-QTOF technology, and the protein was identified by searching the protein database, and it was determined that the receptor of rLZ-8 on the surface of lymphocytes was PSAP.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com