Coordination compound with half-sandwich iridium structure and solid-phase synthesis method
A coordination compound and solid-phase synthesis technology, applied in chemical instruments and methods, organic chemistry, metallocene, etc., can solve problems such as environmental impact, large energy consumption, and harsh reaction conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0046] Synthesis of Complex 1a of Half-Sandwich Iridium and Octahydroxyquinoline Derivatives:
[0047]
[0048] Weigh [Cp*Ir(μ-Cl)Cl] 2 (0.10mmol) placed in a quartz mortar, add octahydroxyquinoline derivative 1 (0.2mmol), then add K 2 CO 3 (0.2mmol), then add 5 drops of CH 2 Cl 2 (0.10 mL). After grinding with an agate mortar for 40 min at room temperature (25°C), add 5 mL of CH 2 Cl 2 Dissolve and filter 2-3 times to remove insoluble matter. Remove most of the CH under reduced pressure using a rotary evaporator 2 Cl 2 , separated by column chromatography (n-hexane:isopropanol=3:1) to obtain the complex 1a of half-sandwiched iridium and octahydroxyquinoline derivatives (90% yield).
[0049] Characterized by proton nuclear magnetic spectrum, carbon spectrum, mass spectrum and infrared. 1 H NMR (400MHz, CDCl 3 , ppm): δ=10.43(s, 1H), 8.11(d, J=8Hz, 1H), 7.92(d, J=8Hz, 1H), 7.51(t, J=16Hz, 1H), 7.04(d, J=8Hz,1H),6.82(d,J=8Hz,1H),1.63(s,15H). 13 C NMR (100MHz, CDC...
Embodiment 2
[0051] Synthesis of Complex 2a of Half-Sandwich Iridium and Octahydroxyquinoline Derivatives:
[0052]
[0053] Weigh [Cp*Ir(μ-Cl)Cl] 2 (0.10mmol) is placed in quartz mortar, adds octahydroxyquinoline derivative 2 (0.2mmol), then adds K 2 CO 3 (0.2mmol), then add 5 drops of CH 2 Cl 2 (0.10 mL). After grinding with an agate mortar for 40 min at room temperature (10°C), add 5 mL of CH 2 Cl 2 Dissolve and filter 2-3 times to remove insoluble matter. Remove most of the CH under reduced pressure using a rotary evaporator 2 Cl 2 , separated by column chromatography (n-hexane:isopropanol=3:1) to obtain the complex 2a of half-sandwiched iridium and octahydroxyquinoline derivatives (88% yield).
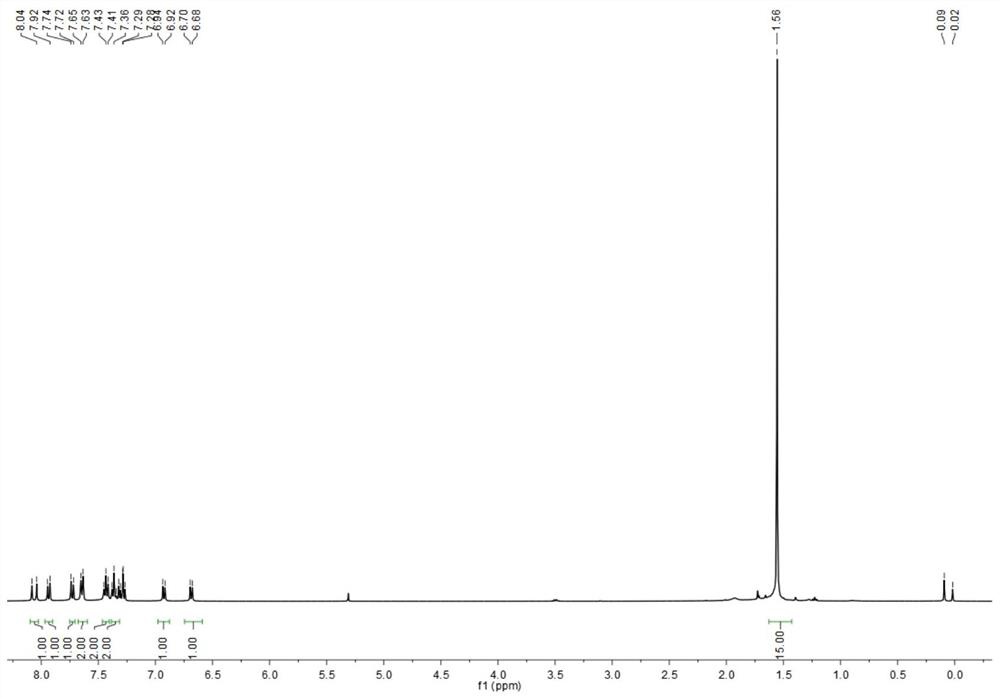
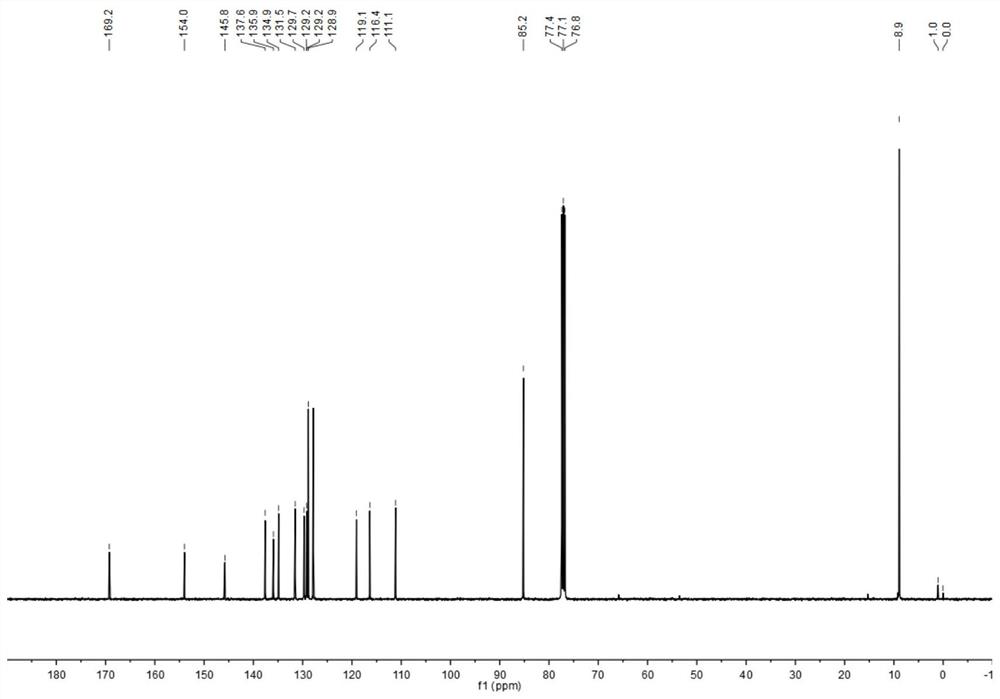
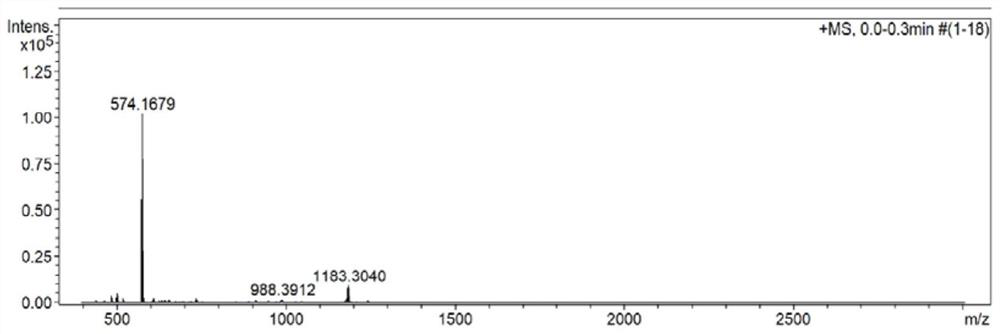
[0054] Characterized by proton nuclear magnetic spectrum, carbon spectrum, mass spectrometry and infrared, respectively see Figure 1-Figure 4 . 1 H NMR (400 MHz, CDCl 3 , ppm): δ=8.04(d, J=16Hz, 1H), 7.92(d, J=12Hz, 1H), 7.74(d, J=8Hz, 1H), 7.65(d, J=8Hz, 2H), 7.43(t, J=16Hz, ...
Embodiment 3
[0056] Synthesis of complex 3a of half-sandwich iridium and octahydroxyquinoline derivatives:
[0057]
[0058] Weigh [Cp*Ir(μ-Cl)Cl] 2 (0.10mmol) placed in a quartz mortar, add octahydroxyquinoline derivative 3 (0.2mmol), then add K 2 CO 3 (0.2mmol), then add 5 drops of CH 2 Cl 2 (0.10 mL). After grinding with an agate mortar for 40 min at room temperature (18°C), add 5 mL of CH 2 Cl 2 Dissolve and filter 2-3 times to remove insoluble matter. Remove most of the CH under reduced pressure using a rotary evaporator 2 Cl 2 , separated by column chromatography (n-hexane:isopropanol=3:1) to obtain the complex 3a of half-sandwiched iridium and octahydroxyquinoline derivatives (92% yield).
[0059] Characterized by proton nuclear magnetic spectrum, carbon spectrum, mass spectrum and infrared. 1 H NMR (400MHz, CDCl 3 , ppm): δ=8.04(d, J=16Hz, 1H), 7.92(d, J=8Hz, 1H), 7.70(d, J=8Hz, 1H), 7.56(d, J=8Hz, 2H), 7.39(d, J=8Hz, 2H), 7.30(t, J=8Hz, 1H), 7.26(d, J=8Hz, 1H), 6.92(d...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com