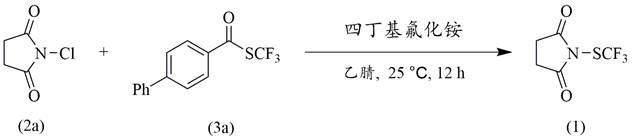Synthetic method of succinimide type trifluoromethyl sulfuration reagent
A succinimide-type and succinimide technology is applied in the field of synthesis of succinimide-type trifluoromethane vulcanization reagents, and can solve the problems that limit the practicability of the synthesis method, affect the accuracy of experimental results, transition metal Element residues and other problems, to achieve the effect of reducing synthesis costs, facilitating industrial production, and mild conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0030] Example 1: In this example, N-chlorosuccinimide and 4-phenylbenzoic acid trifluoromethyl thioester (S-(trifluoromethyl)[1,1'-biphenyl]-4-carbothioate) were used The reaction synthesizes the trifluoromethyl sulfide reagent:
[0031] The reaction equation is:
[0032]
[0033] The synthesis steps and process are as follows: to a 10 mL reaction tube equipped with a magnetic stirring bar, add 4-phenylbenzoic acid trifluoromethyl thioester 3a (0.4 mmol, 112.8 mg), tetrabutylammonium fluoride (0.5 mmol, 130.5 mg), 3.5 mL of acetonitrile, and after mixing, N-chlorosuccinimide 2a (0.4 mmol, 53.4 mg) was added; the reaction tube was fixed on a magnetic stirrer, reacted at 25 °C for 12 hours, and separated and purified after completion. The target product 1 was obtained with a yield of 62%.
[0034] The NMR data of compound (1) are:
[0035] 1 H NMR (600 MHz, Chloroform-d): δ 2.96 (s, 4 H).
[0036] 13 C NMR (151 MHz, Chloroform-d): δ 127.70 (q, J = 313.5 Hz), 28.46
[...
Embodiment 2
[0038] Example 2: In this example, N-bromosuccinimide and 4-phenylbenzoic acid trifluoromethyl thioester (S-(trifluoromethyl)[1,1'-biphenyl]-4-carbothioate) were used The reaction synthesizes the trifluoromethyl sulfide reagent:
[0039] The reaction equation is:
[0040]
[0041]The synthesis steps and process are as follows: to a 10 mL reaction tube equipped with a magnetic stirring bar, 4-phenylbenzoic acid trifluoromethyl thioester 3a (0.4 mmol, 112.8 mg) and potassium fluoride (0.6 mmol, 34.8 mg) were successively added. , 18-crown ether-6 (1.0 mmol, 264 mg), 4.0 mL of trifluorotoluene, N-bromosuccinimide 2b (0.45 mmol, 80.1 mg); the reaction tube was fixed on a magnetic stirrer, in The reaction was carried out at 30° C. for 6 hours, and after completion, the target product 1 was obtained by separation and purification with a yield of 58%.
Embodiment 3
[0042] Example 3: In this example, N-iodosuccinimide and 4-phenylbenzoic acid trifluoromethyl thioester (S-(trifluoromethyl)[1,1'-biphenyl]-4-carbothioate) were used The reaction synthesizes the trifluoromethyl sulfide reagent:
[0043] The reaction equation is:
[0044]
[0045] The synthesis steps and process are as follows: 4-phenylbenzoic acid trifluoromethylthioester 3a (0.4 mmol, 112.8 mg) and silver fluoride (0.45 mmol, 57.2 mg) were successively added to a 10 mL reaction tube equipped with a magnetic stirring bar. , 3.0 mL of acetonitrile, and after mixing, N-iodosuccinimide 2c (0.4 mmol, 90.0 mg) was added; the reaction tube was fixed on a magnetic stirrer, and the reaction was carried out at 50 °C for 24 hours. After completion, the target was separated and purified. Product 1, 45% yield.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com