Composition for treating Alzheimer's disease as well as preparation method and application thereof
A technology for Alzheimer's disease and a composition is applied in the field of compositions for the treatment of Alzheimer's disease, which can solve the problem of Alzheimer's disease treatment, the inability to achieve Alzheimer's disease, and the inability to develop specific therapeutic drugs and other issues to achieve convincing safety, prevention of Alzheimer's symptoms, and clear effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1A
[0031] The establishment of embodiment 1AD experimental animal model
[0032] The model animal strain is SPF-grade SD rats, healthy, male, weighing 250g-300g, provided by Shanghai Nanfang Model Biotechnology Development Co., Ltd. The experimental groups were divided into normal control group, AD model group, positive drug group, test drug group 1, test drug group 2, and test drug group 3, a total of six groups, with 8 to 11 rats in each group.
[0033] In addition to the normal control group, β-amyloid (specifically Aβ 1-40 , purchased from Sigma Company) injected into the hippocampus to induce rats is a modeling method for AD rats. Specifically, rats were anesthetized by intraperitoneal injection of 10% chloral hydrate solution, with a dosage of 4.5ml / kg. SD rats were anesthetized by intraperitoneal injection of 1% pentobarbital sodium 40 μg / g, and then fixed on a brain stereotaxic apparatus after anesthesia. According to the rat brain stereotaxic atlas, the anterior bregm...
Embodiment 2
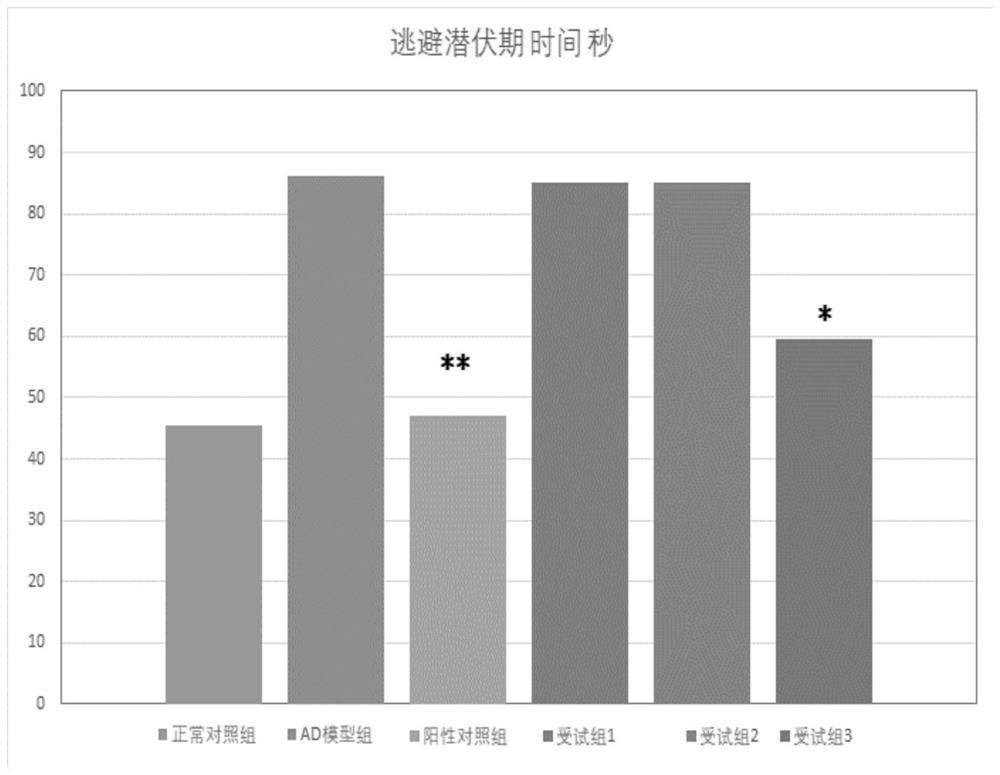
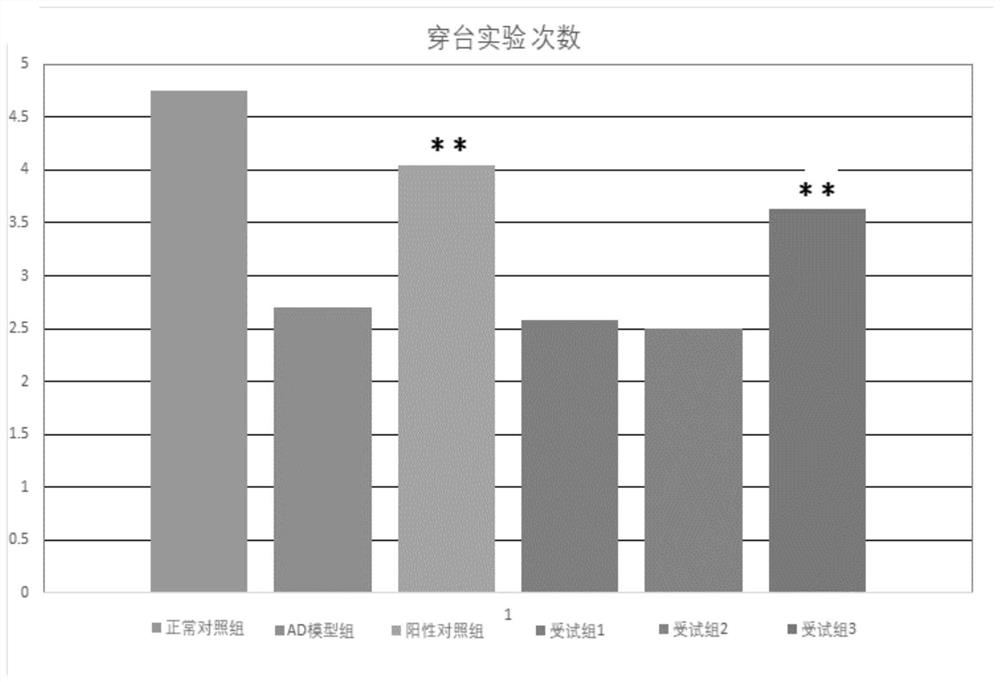
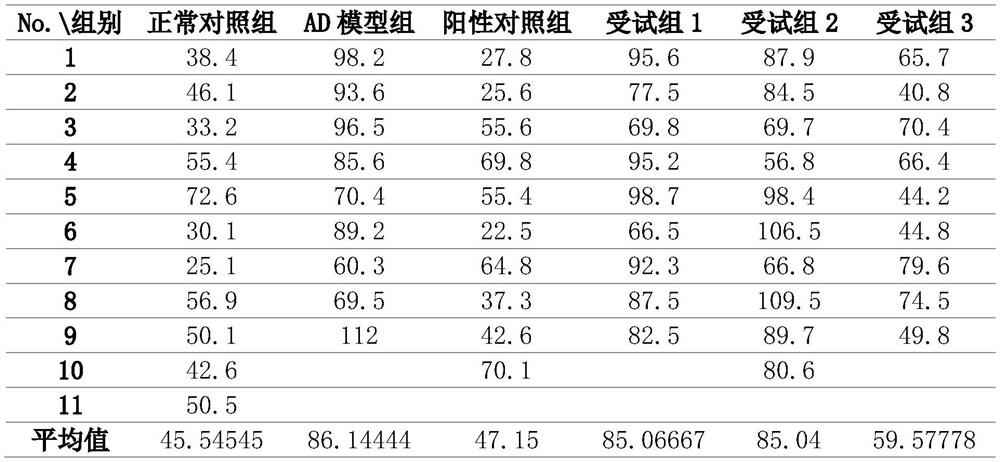
[0034] Embodiment 2 Behavioral detection——Morris water maze model test
[0035] The escape latency test was performed in the first 3 days, and the platform was removed from the platform on the 4th day for the cross-platform test.
[0036] 2.1 Escape latency experiment
[0037] The so-called incubation period experiment means that each rat enters the water from the same position in the test, and the experimenter must assist the rat to enter the water slowly. In order to ensure that the environment for rats to swim on the stage is as similar as possible, pay attention to replenishing water and keep the platform 2cm away from the water surface. After the rat enters the water, start timing, find the platform and stay for 2 seconds, and record the time. The time spent is the incubation period, and the platform has not been found after more than 120 seconds It is also recorded as 120 seconds. The shorter the time, the better the memory improvement effect.
[0038] 2.2 Cross-platf...
PUM
| Property | Measurement | Unit |
|---|---|---|
| weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com