Preparation method and application of fructosazine
A technology of fructosine and fructose, applied in the field of chemical preparations, can solve the problems of high cost, long reaction time, difficult preparation of catalysts and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
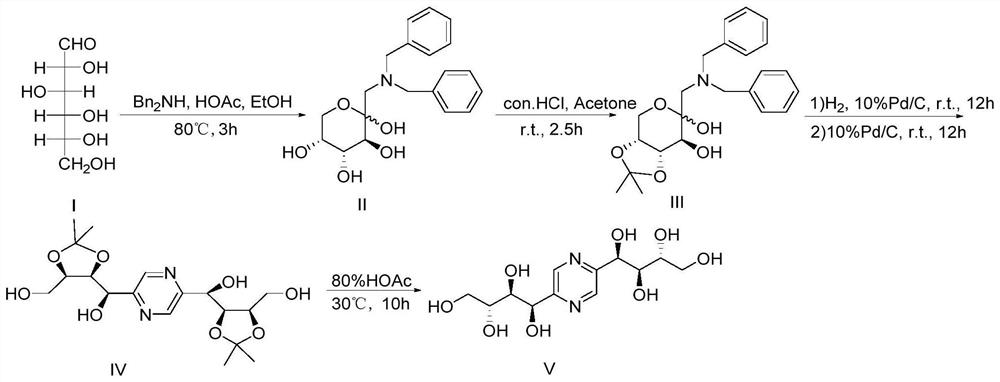
[0038] Embodiment 1: the synthesis of fructozine
[0039] figure 1 For the synthetic route diagram of the preparation method of the fructozine provided in the examples of the present invention, see figure 1 .
[0040] In this embodiment, the synthesis of fructozine comprises the following steps:
[0041] Synthesis of Step 1, 1-dibenzylamino-1-deoxy-D-fructose [20]
[0042] Weigh 3.6g of D-glucose (compound I) (D-glucose, 20mmol) into a three-necked flask, add 40mL of absolute ethanol, stir at room temperature, add 3.96g of dibenzylamine, 1mL of glacial acetic acid in turn, stir evenly, and Reflux at 80°C for 3 hours, cool to room temperature, vacuum filter, wash the filter cake with absolute ethanol 3×10mL and filter with suction to obtain (3S, 4R, 5R)-2-((dibenzylamino)methyl) tetra Hydrogen-2H-pyran-2,3,4,5-tetrol 5.9g, namely 1-dibenzylamino-1-deoxy-D-fructose (compound II), yield 82%. 1 H NMR (400 MHz, DMSO-d6) δ7.30 (m, 10H, Ph-H), 5.25 (s, 1H, OH), 4.43 (d, J=4.7...
Embodiment 2
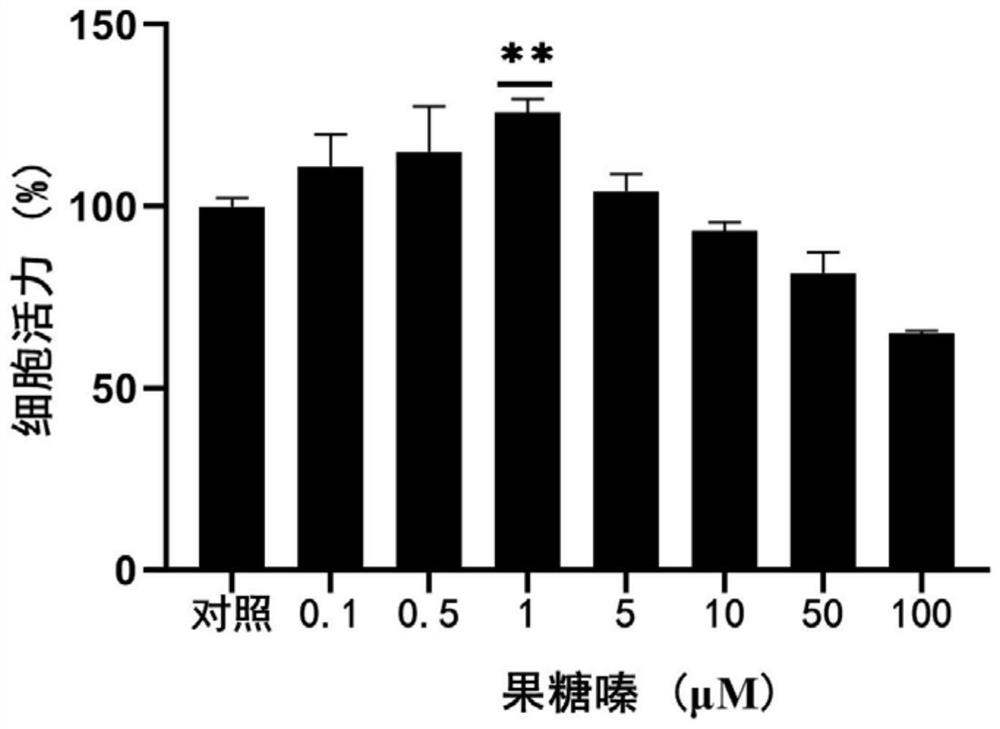
[0054] Embodiment 2: Anti-aging activity evaluation of fructozine
[0055] 1. Experimental method
[0056] 1. Cell culture
[0057] The 2BS cell line isolated from human fetal lung fibroblasts was originally established by the China Institute of Biological Products and has been widely used as a model of cellular senescence. It is generally believed that PD (population doubling, population doubling times, also known as "generations") below 30 are young cells, while replicative senescent cells are PD55 or above. The cells in this experiment were purchased from the China Type Culture Collection Center of Wuhan University. The cells were grown in MEM supplemented with 10% fetal bovine serum, 1% penicillin and streptomycin double antibody solution, and placed at 37°C, 5% CO 2 in the incubator. When the cells grew to 85%, the cultured cells were split and passaged at a ratio of 1:2 or 1:4. Cumulative cell doublings (CPDs) were calculated as log 2 (D / D 0 ), where D and D 0 are...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com