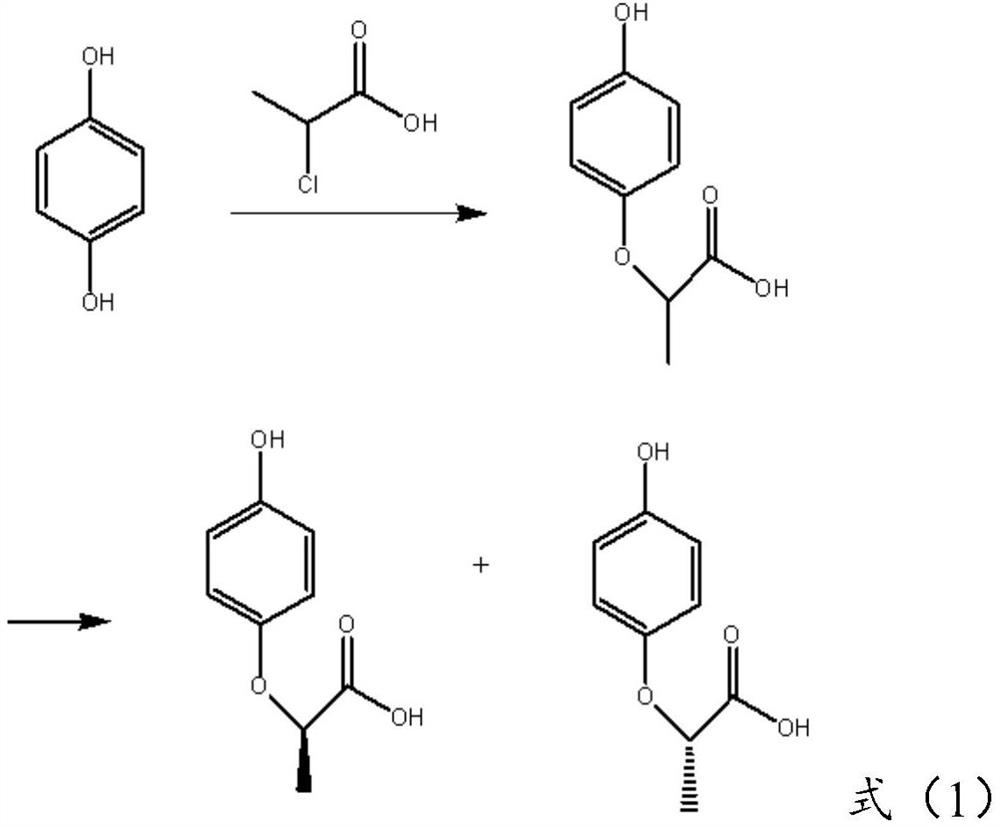
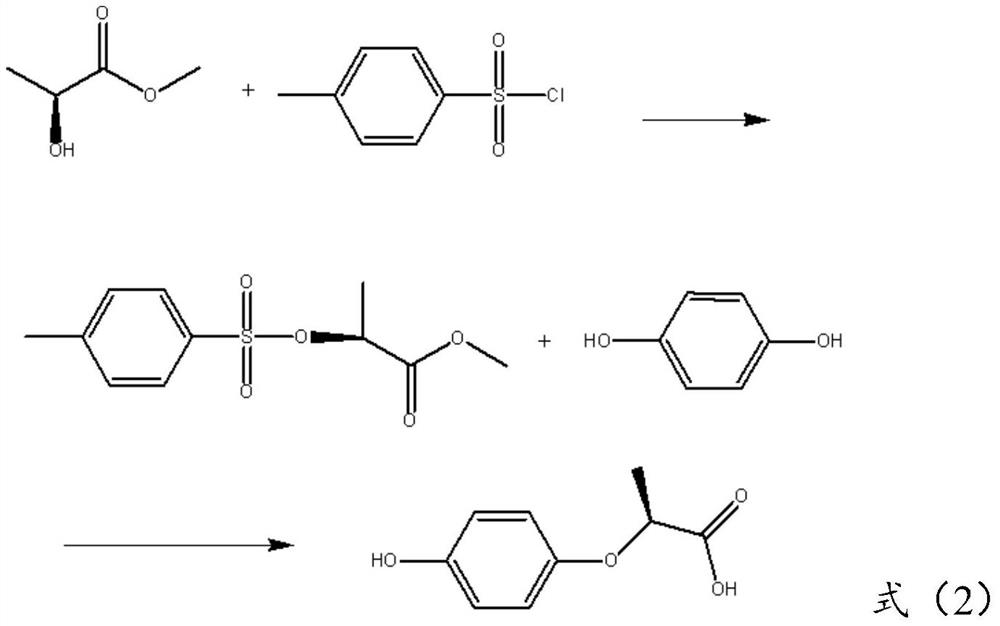
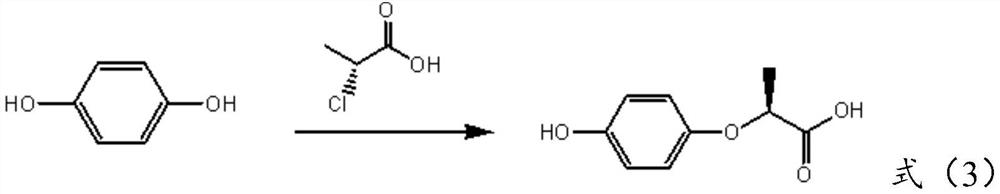
Preparation method of (R)-(+)-2-(4-hydroxyphenoxy) propionic acid
A technology of hydroxyphenoxy and chlorophenoxy, applied in the field of electric power environmental protection, can solve the problems of difficult purification of products, large environmental pollution, low yield, etc., and achieve the effect of low price and simple process operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0028] The invention provides a method for preparing (R)-(+)-2-(4-hydroxyphenoxy)propionic acid, comprising the following steps:
[0029] Condensation reaction of phenolic compound and (S)-(-)-2-halopropionic acid under basic conditions to obtain R-(+)-2-(4-chlorophenoxy)propionic acid; the Phenolic compounds are p-chlorophenol, p-bromophenol, p-iodophenol or p-hydroxybenzenesulfonic acid;
[0030] The R-(+)-2-(4-chlorophenoxy)propionic acid is hydrolyzed under the catalysis of a catalyst to obtain the (R)-(+)-2-(4-hydroxyphenoxy ) Propionic acid.
[0031] In the present invention, unless otherwise specified, the raw materials used are commercially available products in the art.
[0032] In the present invention, phenolic compounds and (S)-(-)-2-halopropionic acid are condensed under alkaline conditions to obtain R-(+)-2-(4-chlorophenoxy)propionic acid; The phenolic compound is p-chlorophenol, p-bromophenol, p-iodophenol or p-hydroxybenzenesulfonic acid.
[0033] In the pr...
Embodiment 1
[0057] (1) Synthesis of R-(+)-2-(4-chlorophenoxy)propionic acid: Add 128.6g of p-chlorophenol, 200mL of 30% mass concentration sodium hydroxide aqueous solution in a 1L four-necked bottle, and heat up to Stir at 50°C for 30min, add 108.5g of (S)-(-)-2-chloropropionic acid dropwise, keep the reaction at 50°C for 2 hours, and perform liquid phase detection (Shimadzu liquid chromatography, detection condition: C18 chromatographic column, wavelength 230nm , column temperature 30°C, mobile phase acetonitrile and water volume ratio = 15:85) raw material p-chlorophenol HPLC area normalized content < 0.3%, concentrated hydrochloric acid to adjust pH = 1, filter off-white solid R-(+)- 200.2 g of 2-(4-chlorophenoxy)propionic acid (water content 10wt% by Karl Fischer test), liquid phase content (area normalized content) = 97.4%, directly proceed to the next step without drying.
[0058] (2) Synthesis of R-(+)-2-(4-hydroxyphenoxy)propionic acid: add 200.2 R-(+)-2-(4-chlorophenoxy)propioni...
Embodiment 2
[0064] (1) Synthesis of R-(+)-2-(4-chlorophenoxy)propionic acid: 128.6g of p-chlorophenol was added in a 1L four-necked bottle, 200mL of 35% mass concentration sodium hydroxide aqueous solution was heated to Stir at 60°C for 30min, add 108.5g of (S)-(-)-2-chloropropionic acid dropwise, keep the reaction at 60°C for 2 hours, and perform liquid phase detection (Shimadzu liquid chromatograph, detection condition: C18 chromatographic column, wavelength 230nm , column temperature 30°C, mobile phase acetonitrile and water volume ratio = 15:85) raw material p-chlorophenol HPLC area normalized content < 0.3%, concentrated hydrochloric acid to adjust pH = 1, filter off-white solid R-(+)- 200.8g of 2-(4-chlorophenoxy)propionic acid (water content 10wt% by Karl Fischer test), liquid phase content (area normalized content) = 97.2%, directly proceed to the next step without drying.
[0065] (2) Synthesis of R-(+)-2-(4-hydroxyphenoxy)propionic acid: add 200.8 R-(+)-2-(4-chlorophenoxy)propio...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com