Isoindoline derivative compound, preparation method and weight losing application
An isoindoline and compound technology, applied in the field of medicine, can solve the problems of small toxic and side effects, good absorption, adverse reactions, etc., and achieve the effects of reducing body weight, reducing liver fat and epididymal fat content, and improving development prospects.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
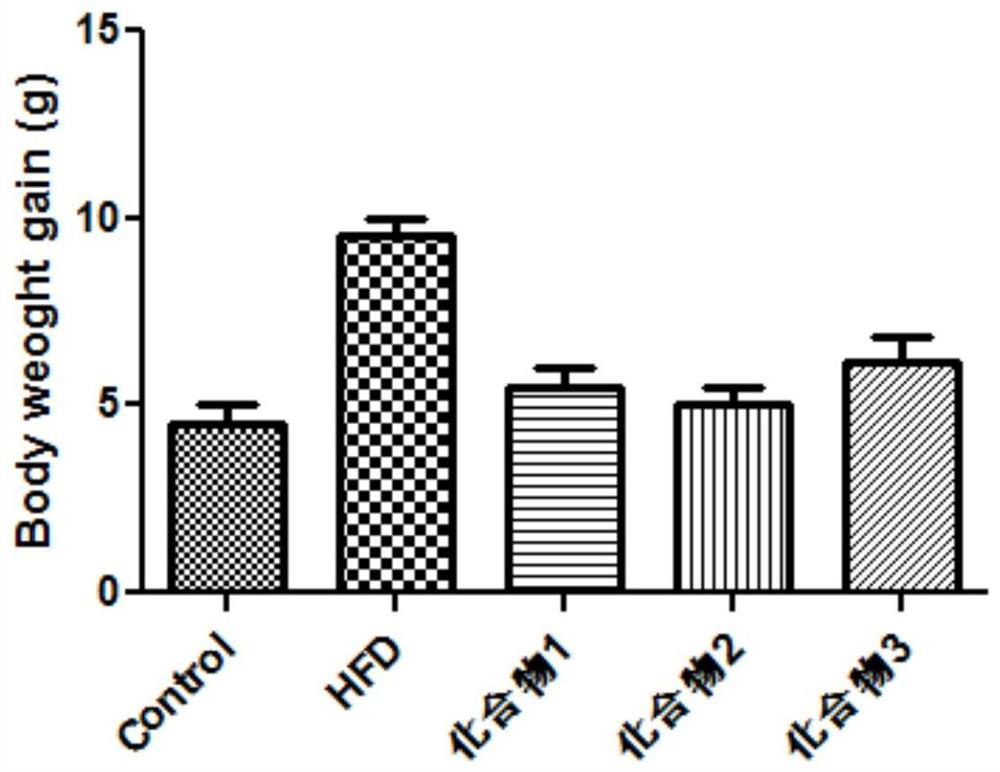
Examples
Embodiment 1
[0026] Embodiment 1, the preparation of compound 1
[0027] (1) Synthesis of 2-(2-nitro-ethyl) isoindoline:
[0028]
[0029] At 0-5°C under a nitrogen atmosphere, add isoindoline (7.1 g, 59.58 mmol), cuprous bromide (7.2 g, 50.19 mmol), triethylamine (2.0 g, 19.76 mmol) and Tetrahydrofuran (50 mL) was added dropwise with 1-bromo-2-nitroethane (18.5 g, 120.16 mmol) under stirring. After the drop was completed, the temperature was slowly raised to 30-35° C., stirred for 36 hours, and the reaction was detected by TLC. Cool to room temperature, remove insoluble matter by filtration, recover the solvent under normal pressure, and distill under reduced pressure to obtain light yellow liquid 2-(2-nitro-ethyl)isoindoline, 9.46g, yield 82.6%.
[0030] 1 H-NMR (400MHz, CDCl 3 )δ:3.11(t,2H),3.79(s,4H),4.63(t,1H),4.96(t,1H),7.33-7.46(m,4H). 13 C-NMR (125MHz, CDCl 3 )δ: 52.73, 60.97, 71.57, 124.78, 127.96, 139.81. LC-MS (ESI, pos, ion) m / z: 193.09[M+H].
[0031] (2) Synthesis of ...
Embodiment 2
[0042] The synthesis of embodiment 2, compound 2
[0043]
[0044] 2-Amino-3-(isoindolin-2-yl)-1-(4-methoxyphenyl)propan-1-ol (14.92g, 50.00mmol), 2-chloro-N-( 4-Ethoxy-3-methoxyphenethyl)acetamide (18.20g, 66.99mmol), sodium iodide (9.74g, 65.00mmol) and potassium carbonate (14.51g, 105.00mmol) were added to dimethylformaldehyde amide (120 mL) and stirred at 50°C for 80 hours. The solvent was distilled off and the crude product was dissolved in water (120 mL) and extracted with dichloromethane (4 x 30 mL). The combined organic phases were dried over anhydrous sodium sulfate, filtered and concentrated under reduced pressure to remove the solvent. The crude product was purified by column chromatography (eluent: EA:acetone=1:1). N-(4-ethoxy-3-methoxyphenethyl)-2-((1-hydroxy-3-(isoindolin-2-yl)-1-(4- Methoxyphenyl)propan-2-yl)amino)acetamide (compound 2), 17.48g, yield 65.5%.
Embodiment 3
[0045] The synthesis of embodiment 3, compound 3
[0046]
[0047] 2-Amino-3-(isoindolin-2-yl)-1-(4-methoxyphenyl)propan-1-ol (14.92g, 50.00mmol), 2-chloro-N-( 3,4-Diethoxyphenethyl)acetamide (19.14g, 66.99mmol), sodium iodide (9.74g, 65.00mmol) and potassium carbonate (14.51g, 105.00mmol) were added to dimethylformamide (120mL ) and stirred at 50°C for 80 hours. The solvent was distilled off and the crude product was dissolved in water (120 mL) and extracted with dichloromethane (4 x 30 mL). The combined organic phases were dried over anhydrous sodium sulfate, filtered and concentrated under reduced pressure to remove the solvent. The crude product was purified by column chromatography (eluent: EA:acetone=1:1). N-(3,4-diethoxyphenethyl)-2-((1-hydroxy-3-(isoindolin-2-yl)-1-(4-methoxy Phenyl)propan-2-yl)amino)acetamide (compound 3), 21.52g, yield 78.6%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com