Heterocyclic compound
A technology of compounds and heterocycles, applied in the direction of active ingredients of heterocycles, organic chemistry, drug combination, etc., can solve problems such as shortage of platelet preparations
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment approach
[0081] (1) In formula [I], ring A is benzene or pyridine, and the hydrogen on the ring of benzene or pyridine is optionally substituted by fluorine, methyl or -CN;
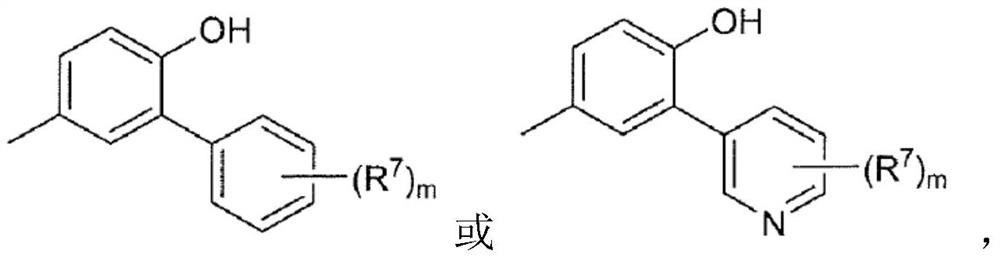
[0082] (2) In formula [I], ring B is represented by the following formula:
[0083] [chemical formula 3]
[0084]
[0085] where R 7 is halogen, C optionally substituted by halogen 1-6 Alkyl, -CN, -OR 5 、-SR 5 、-COOR 5 、-C 1-6 Alkylene-CONR 5 R 6 、-C 1-6 Alkylene-OCOR 5 ,-CONR 5 R 6 , -NR 5 COOR 6 、-SO 2 R 5 or -NR 5 R 6 (R 5 and R 6 the same or different and each independently represents hydrogen or C optionally substituted by halogen 1-6 alkyl),
[0086] m is 0, 1 or 2, when m is 2, each R 7 are independently the same or different substituents,
[0087] For example, (R 7 )m represents fluorine, two fluorine, chlorine, methyl, ethyl, trifluoromethyl, hydroxyl, methoxy, -CN, -CON(CH 3 ) 2 、-CH 2 CON(CH 3 ) 2 , methylsulfonyl, or fluorine and -CN are each substituted;
[0088] (3) In ...
Embodiment
[0226] The present invention is explained in detail below by referring to Test Examples, Reference Examples and Examples, which are not construed as limiting, and the present invention can be changed within the scope of the present invention.
[0227] In this specification, the following abbreviations may be used.
[0228]
[0229]
[0230]
[0231] In the following examples, "room temperature" generally refers to about 10°C to about 35°C. The ratios indicated for mixed solvents are volume mixing ratios unless otherwise stated. % are by weight unless otherwise stated.
[0232] 1 HNMR (proton nuclear magnetic resonance spectrum) was measured by Fourier-transform NMR (Bruker AVANCE III 400 (400 MHz) or Bruker AVANCE III HD (500 MHz)).
[0233] Mass spectra (MS) were determined by LC / MS (ACQUITY UPLC H-Class). As the ionization method, the ESI method is used. The data represent actually measured values (actually measured values). Typically, molecular ion peaks ar...
reference example 1
[0238] Synthesis of 2-amino-6-chloro-9-(1-hydroxy-2-methylpropan-2-yl)-7H-purin-8-one
[0239] A solution of 2,5-diamino-4,6-dichloropyrimidine (10.0 g) and 2-amino-2-methyl-1-propanol (11.7 ml) in NMP (10 ml) was stirred overnight at 140°C. The reaction mixture was purified by column chromatography (Hexane / AcOEt / MeOH). To a solution of the product in THF (150ml) was added CDI (19.9g) at 0°C, and the mixture was stirred for 1 hr. To the mixture were added 50% aqueous MeOH (300 ml) and 5N aqueous NaOH (44.7 ml), and the mixture was stirred for 1 hr. The reaction mixture was concentrated, 5N aqueous HCl solution was added to the residue, and the solid precipitate was collected by filtration to obtain the title compound (10.9 g).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com