A kind of crystalline form of opioid receptor (mor) agonist and preparation method
A crystal form and drug technology, which is applied in the field of crystallization and preparation of opioid receptor (MOR) agonists, can solve the problems of poor product stability, poor fluidity, and easy agglomeration, and achieve stable production process and high solubility. Good effect with good crystal stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
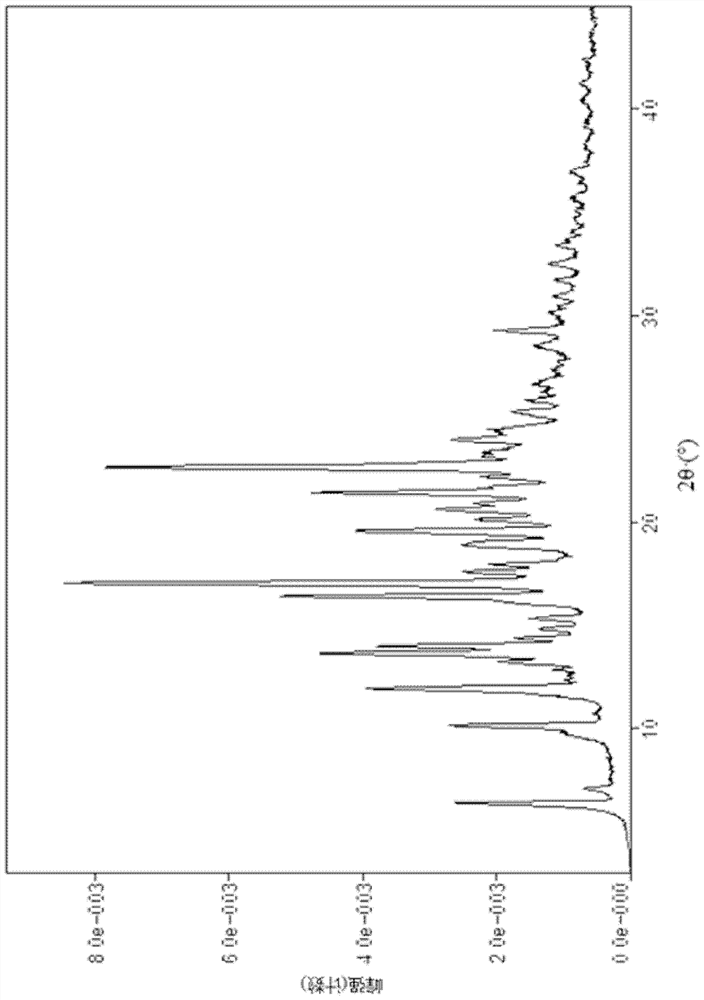
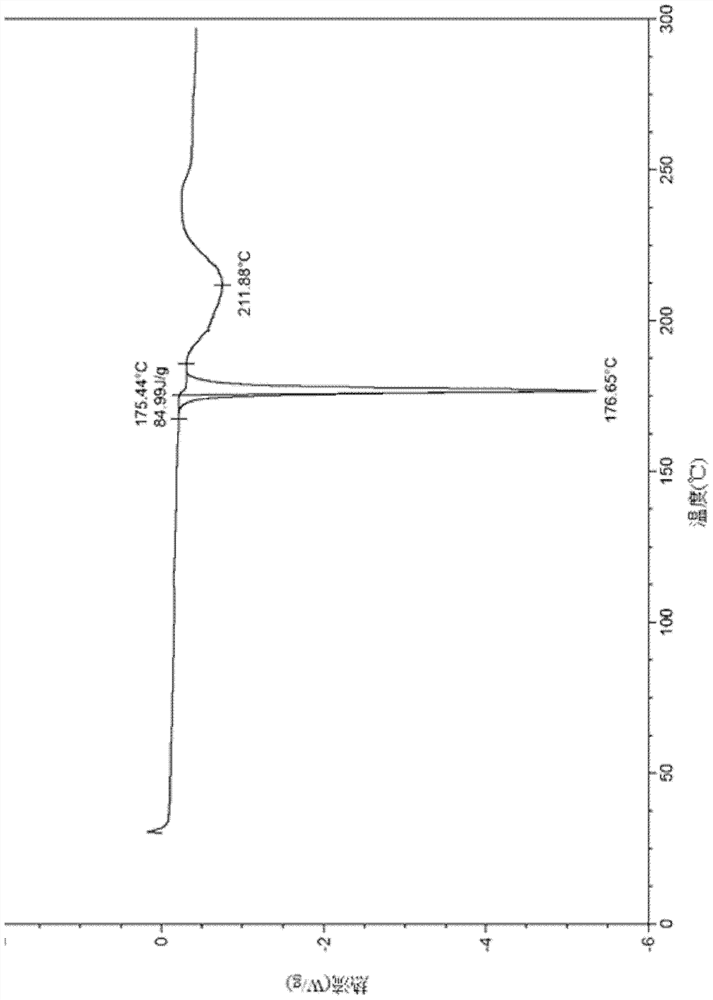
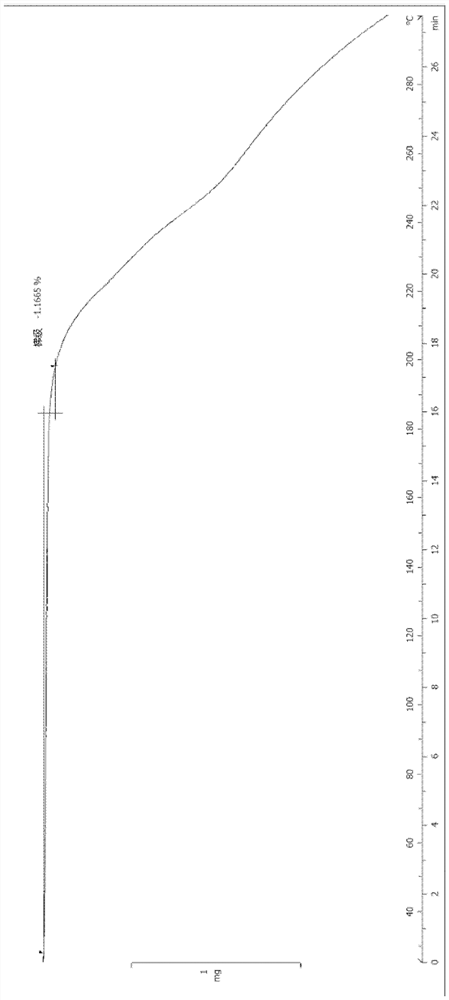
[0094] (1S, 4S)-4-ethoxy-N-(2-((R)-9-(pyridin-2-yl)-6-oxaspiro[4.5]dec-9-yl)ethyl)- Preparation of 1,2,3,4-tetrahydronaphthalene-1-amine fumarate (III crystal form)
[0095]
[0096] (1S, 4S)-4-ethoxy-N-(2-((R)-9-(pyridin-2-yl)-6-oxaspiro[4.5]dec-9-yl)ethyl) -1,2,3,4-Tetralin-1-amine (2.6g, 5.96mmol) was dissolved in isopropanol (10mL), heated to 80°C, fumaric acid (695mg, 5.96mmol) and isopropanol Propanol (10 mL) was added to another reaction flask, heated to 80°C and stirred to dissolve, then added dropwise to the above solution, refluxed and stirred for 10 minutes, cooled naturally to 40°C, a white solid precipitated in the solution, and then cooled to room temperature, The reaction was stirred for 1.5 hours. The reaction solution was filtered, and the filter cake was rinsed with isopropanol (2mL×5) and ethyl acetate (2mL×3) successively. The filter cake was collected and vacuum-dried to obtain a white solid product (2.0g, yield 60%). The XRPD pattern of the sample i...
Embodiment 2、II
[0103] Embodiment 2, investigation on stability of influencing factors of III crystal form
[0104] The III crystal sample obtained in Example 1 was placed open and flat, and the chemical stability of the sample was investigated under the conditions of light (4500Lux), high temperature (40°C, 60°C), and high humidity (RH75%, RH90%). The sampling time is 10 days and 17 days, and the chemical purity and chiral purity of the samples are investigated, and the HPLC detection purity is shown in the table below.
[0105] test results:
[0106] Table 2, Stability of Influencing Factors of Form III Crystalline Samples (HPLC Purity)
[0107]
[0108] Test Conclusions:
[0109] The crystal form III was kept open for 17 days under the conditions of light and high temperature (40°C, 60°C), and the chemical purity and chiral purity decreased significantly. Purity and chiral purity have little change; XRPD detection of crystal form III was placed under light (4500Lux), high temperature...
Embodiment 3、II
[0110] Embodiment 3, long-term and accelerated stability investigation of III crystal form
[0111] The crystal form III sample obtained in Example 1 was protected from light, sealed and laid flat to investigate the stability of the sample under long-term (25°C, 60%RH) and accelerated (40°C, 75%RH), and the sampling time was 0.5 Month, 1 month, 2 months, 3 months, XRPD detects whether the crystal form changes.
[0112] test results:
[0113] The stability (HPLC purity) of table 3, III crystal form sample
[0114]
[0115] Test Conclusions:
[0116] The crystal form III has good stability under long-term (25°C, 60% RH) and accelerated (40°C, 75% RH) conditions for 3 months in the dark and sealed condition, and 3 months at 25°C and 60% RH XRPD pattern see Figure 4 , 40 ℃, 75% RH placed 3 months XRPD pattern see Figure 5 , the XRPD peak shape of the III crystal form basically does not change, and the crystal form is stable.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com