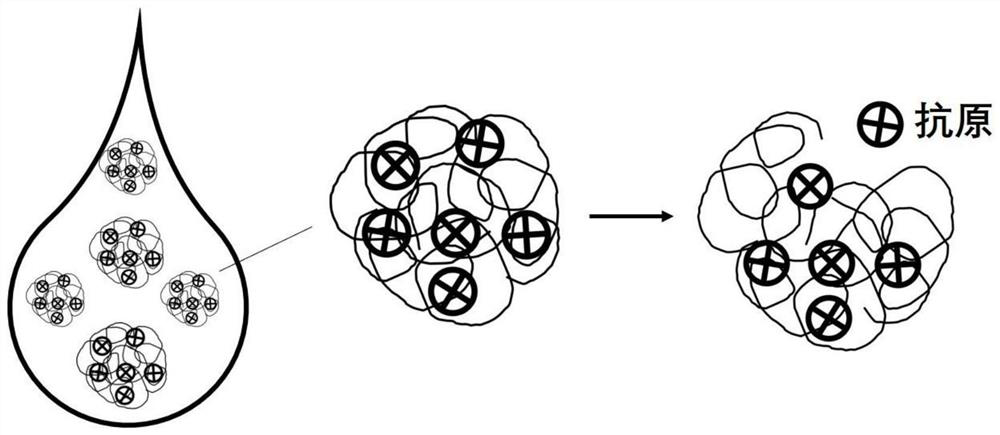
Swine oral inoculation vaccine slow-release adjuvant and preparation method and application thereof
A technology of vaccination and sustained release, applied in the direction of vaccines, veterinary vaccines, pharmaceutical formulations, etc., can solve the problems of unrealistic production and application, lack of immunity, disease in clean pigs, etc., to promote green breeding, and the synthesis process is simple and easy. The effect of line and operation is convenient
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0046] Weigh 0.02g of poly 2-vinylpyridine (molecular weight is about 57kDa), dissolve it in 0.5mL of absolute ethanol to prepare a polymer ethanol solution with a mass concentration of 0.04g / mL. Disperse the inactivated Haemophilus parasuis vaccine in deionized water to prepare an aqueous antigen solution with a concentration of 1 mg / mL. Under the condition of continuous stirring, in the 0.5mL poly 2-vinylpyridine ethanol solution, dropwise add 5mL Haemophilus parasuis inactivated vaccine dispersion liquid with the flow rate of 1mL / min, after the two are stirred and mixed evenly, in 4 Incubate for 4 hours at °C. After the incubation is completed, filter the product mixture through a 0.45 μm filter membrane, collect the filtrate, purify and collect the polymer-antigen nanomicelles through a high-performance liquid chromatography system, and freeze-dry at -18°C for 12 hours to completely remove the solvent The poly 2-vinylpyridine vaccine slow-release adjuvant loaded with inac...
Embodiment 2
[0049] Weigh 0.01 g of poly-4-vinylpyridine (molecular weight is about 60 kDa), dissolve it in 0.5 mL of absolute ethanol to prepare a polymer ethanol solution with a mass concentration of 0.02 g / mL. Disperse the bivalent inactivated vaccine of Streptococcus suis in deionized water to prepare an aqueous antigen solution with a concentration of 1 mg / mL. In the case of continuous stirring, add 3 mL of Streptococcus suis bivalent inactivated vaccine dispersion solution dropwise to 0.5 mL of poly-4-vinylpyridine ethanol solution at a flow rate of 1 mL / min, stir and mix the two evenly, Incubate for 4 hours. After the incubation is completed, filter the product mixture through a 0.45 μm filter membrane, collect the filtrate, purify and collect the polymer-antigen nanomicelles through a high-performance liquid chromatography system, and freeze-dry at -18°C for 12 hours to completely remove the solvent to obtain the poly-4-vinylpyridine vaccine slow-release adjuvant loaded with bival...
Embodiment 3
[0052] Weigh 0.025g of poly-4-vinylpyridine (molecular weight is about 62kDa), dissolve it in 0.5mL of absolute ethanol to prepare a polymer ethanol solution with a mass concentration of 0.05g / mL. Disperse the inactivated Haemophilus parasuis vaccine in deionized water to prepare an aqueous antigen solution with a concentration of 1 mg / mL. In the case of continuous stirring, add 8 mL of Haemophilus parasuis inactivated vaccine dispersion solution dropwise at a flow rate of 0.5 mL / min to 0.5 mL of poly-4-vinylpyridine ethanol solution, stir and mix the two, and then To the mixture, 0.005 mL (4 mg) of peanut oil was added dropwise at a flow rate of 0.5 mg, stirred evenly, and incubated at 4° C. for 3 hours. After the incubation is completed, filter the product mixture through a 0.45 μm filter membrane, collect the filtrate, purify and collect the polymer-antigen nanomicelles through a high-performance liquid chromatography system, and freeze-dry at -18°C for 12 hours to complete...
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
| molecular weight | aaaaa | aaaaa |
| concentration | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com