Risperidone and 9-hydroxyrisperidone hapten, antigen and antibody and application thereof
A technology of hydroxyrisperidone and risperidone, which is applied in the field of immunology detection, can solve the problems of cumbersome operation, radioactive pollution, high blood drug concentration, etc., and achieve the effect of simple operation, high specificity and strong specificity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
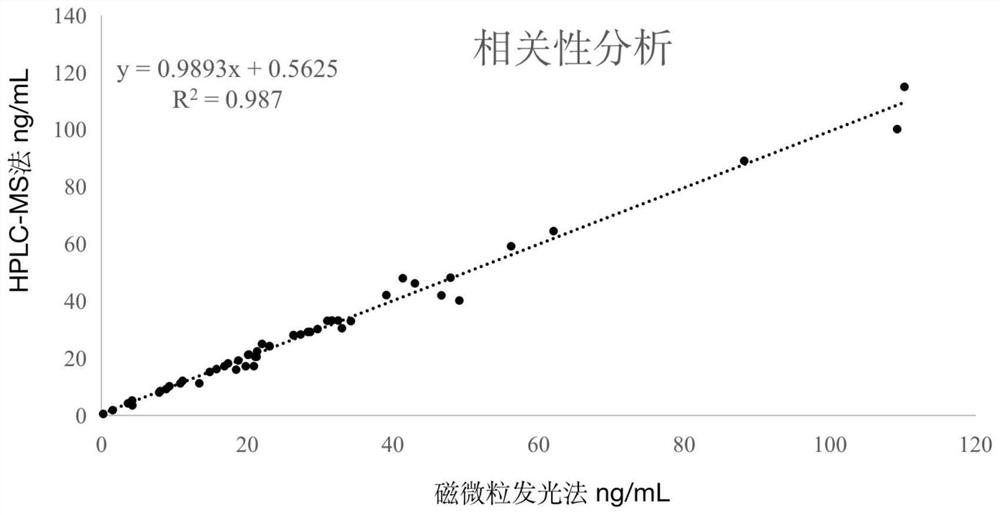
Image
Examples
Embodiment 1
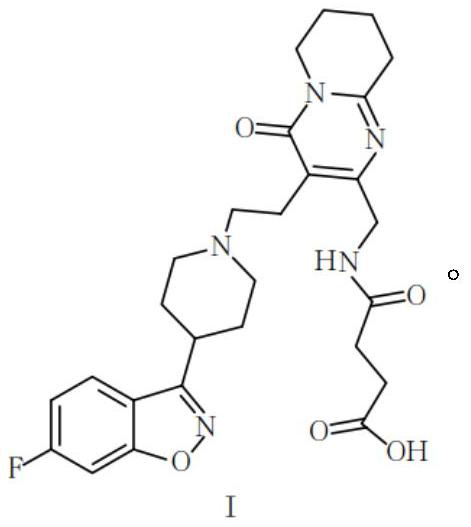
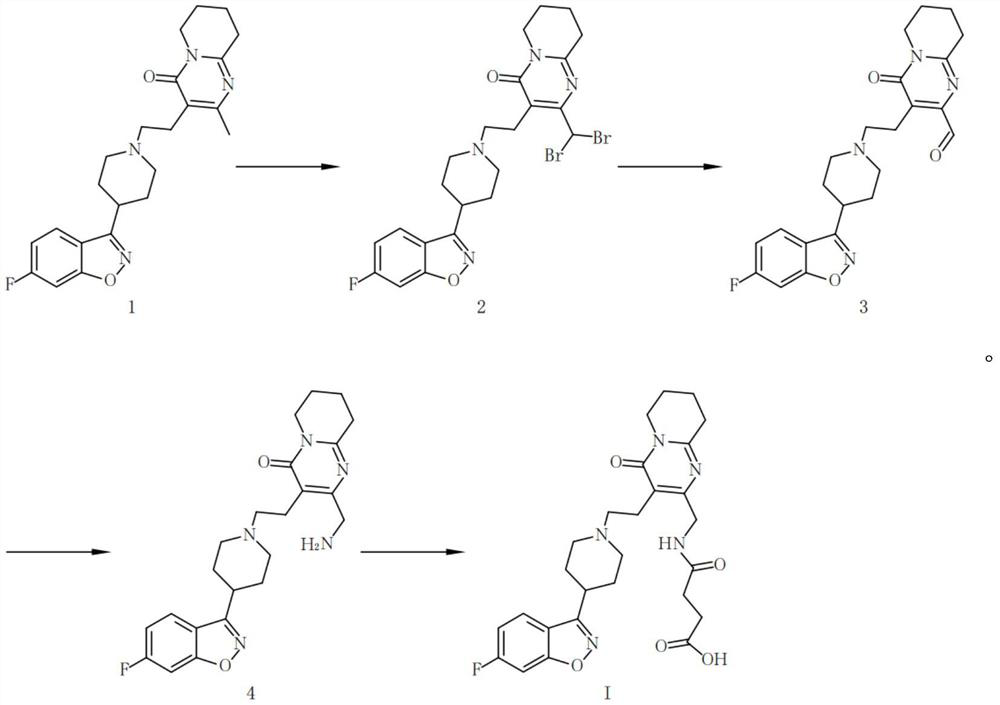
[0063] The structure of the hapten of risperidone and 9-hydroxyrisperidone is shown in formula I, and the specific synthetic route of the compound of formula I is as follows:
[0064]
[0065] Preparation of Compound 2: Add risperidone (4.1g, 0.01mol) into a 250mL one-necked bottle, add 150mL carbon tetrachloride, benzoyl peroxide (0.24g, 0.001mol) and NBS (3.9g, 0.022mol ), the system was refluxed in the dark for 6 hours, the TLC detection reaction was completed, and the system was down to room temperature. Pour the reaction solution into 200mL water for extraction, separate the organic phase, extract the aqueous phase three times with dichloromethane, each time the amount of dichloromethane is 50mL, combine the organic phases, dry over anhydrous sodium sulfate, filter, and evaporate to dryness to obtain a crude product . The crude product was purified by column chromatography to obtain 1.8 g of compound 2 (yield 32%).
[0066] Preparation of compound 3: Add compound 2 (...
Embodiment 2
[0074] Risperidone and 9-hydroxy risperidone antigen, the specific preparation method is as follows:
[0075] (i) dissolving the hapten of 10 mg risperidone and 9-hydroxy risperidone in 1 mL of dimethyl sulfoxide to obtain a dimethyl sulfoxide solution of the hapten;
[0076] (ii) Dissolve 10 mg of 1-ethyl-(3-dimethylaminopropyl) carbodiimide hydrochloride in 100 uL of water to obtain 1-ethyl-(3-dimethylaminopropyl) Aqueous solution of carbodiimide hydrochloride;
[0077] (iii) adding an aqueous solution of 1-ethyl-(3-dimethylaminopropyl)carbodiimide hydrochloride to the dimethyl sulfoxide solution of the hapten, and reacting at room temperature for 1 hour to obtain a reaction solution;
[0078] (iv) the bovine serum albumin of 10mg is dissolved in the PBS damping fluid of 5mL to obtain bovine serum albumin solution;
[0079] (v) mixing the bovine serum albumin solution and the reaction solution, and stirring at room temperature for 2 hours to obtain a reaction mixture;
[...
Embodiment 3
[0083] An antibody capable of binding to risperidone and / or 9-hydroxyrisperidone, the specific preparation method is as follows:
[0084] (i) Selecting a host for antibody production: among them, the host can be mice, rabbits, goats, sheep, etc., and mice are used as hosts in this embodiment;
[0085] (ii) Inoculate the host with risperidone and 9-hydroxyrisperidone antigen: Dilute risperidone and 9-hydroxyrisperidone antigen to 1 mg / mL with physiological saline, add an equal volume of Freund's complete adjuvant, The emulsification was complete, and the mice were immunized for the first time according to the dose of 0.1mg / only; after an interval of four weeks, 1mg of risperidone and 9-hydroxyrisperidone antigen and 1mg of Freund's incomplete adjuvant were mixed and stirred at a speed of 2000rpm / min under the condition of stirring for 2 hours to complete the emulsification, and the mice immunized for the first time were boosted with a dose of 0.1 mg / mouse;
[0086] (iii) The ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com