Special enzyme for galactooligosaccharide production as well as preparation and application thereof
A lactase and recombinant strain technology, applied in the field of enzyme engineering, can solve the problems of difficulty in separation and purification, increase the difficulty of preparing lactase enzyme preparations, and difficult to realize the secretion and expression of lactase, and achieve the effect of reducing the cost of fermentation and manufacturing
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0069] 10. Preparation method of lactase enzyme preparation
[0070] After the fermentation is finished, the bacterial cells are removed by plate and frame filtration, and then the enzyme liquid is obtained after filtration by an ultrafiltration system.
[0071] 11. Determination of lactase activity
[0072] The enzyme activity assay of lactase was improved according to the national standard GB / T 33409-2016. In general, the reaction is carried out at pH 5.0 and 40°C with lactose as substrate. Glucose release was measured using a biosensor.
[0073] The enzyme activity of lactase is defined as the amount of enzyme required to decompose lactose to produce 1 micromole of glucose per minute at pH 5.0 and 40°C, which is defined as an enzyme activity unit (U), expressed in U / mL or U / g.
[0074] 12. Synthesis and product analysis of galactooligosaccharides
[0075] Use 300g / L-800g / L lactose as the substrate, add 5U / g-20U / g lactase, carry out the reaction at 50°C-70°C, and take sa...
Embodiment 1
[0085] Example 1: Molecular evolution of lactase
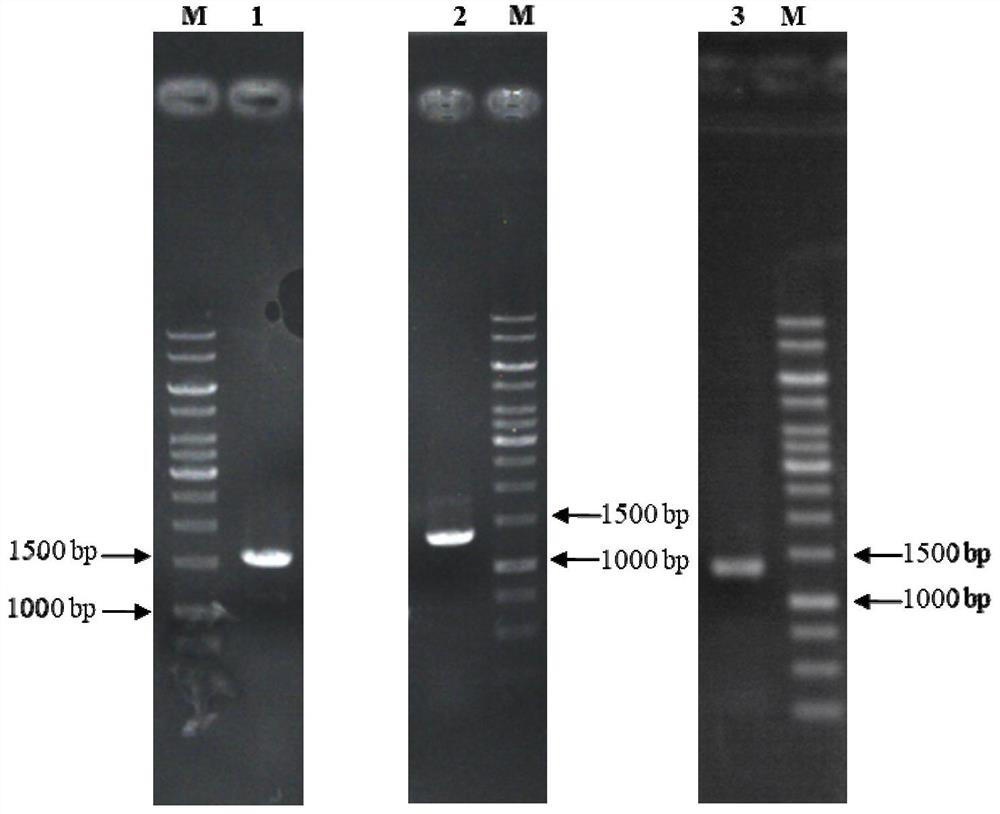
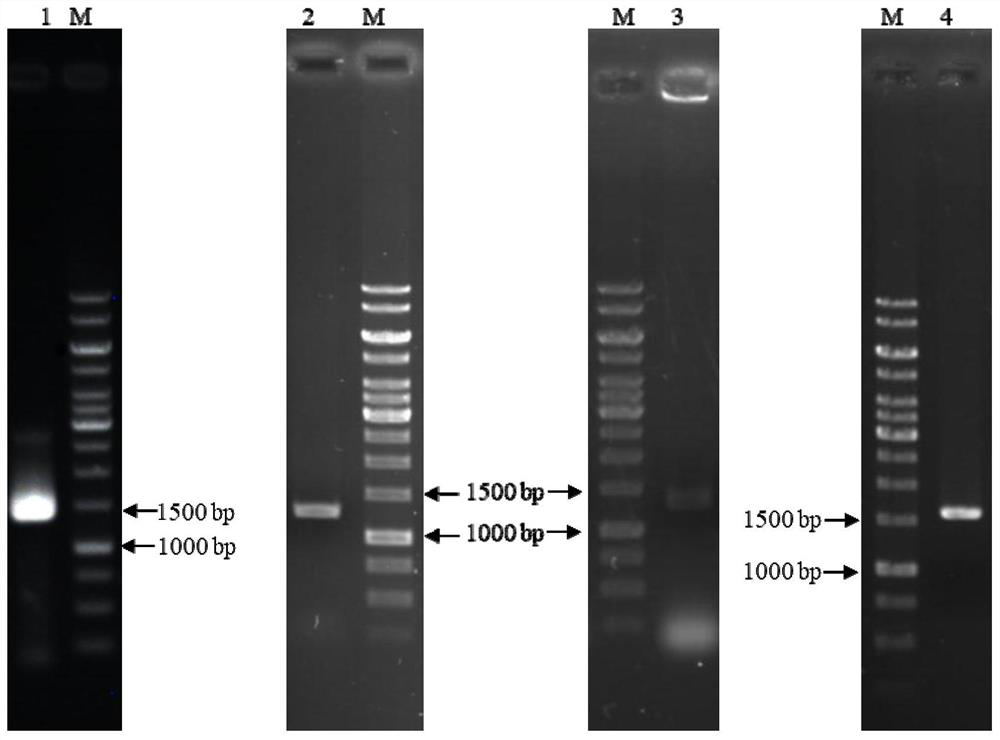
[0086] Using the BglD305 and BglD coding genes shown in SEQ ID NO.1 and SEQ ID NO.7 in the sequence table as templates, the DNA shuffling method was used to carry out molecular evolution. After enzyme activity screening, the lactase enzyme molecule BcBG168 (nucleotide sequence SEQ ID NO.13) with significantly improved enzyme activity level and its amino acid sequence (amino acid sequence SEQ ID NO.14) were obtained.
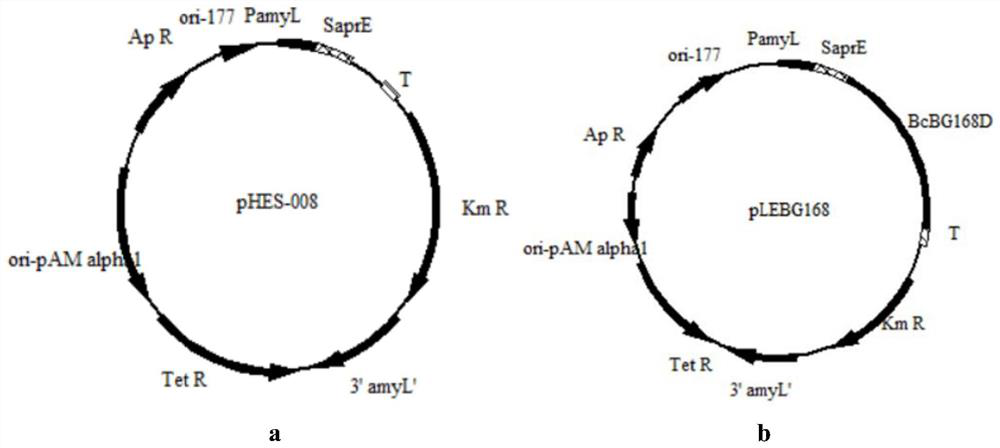
[0087] By truncating the coding genes of BglD305, BglD and BcBG168 to different degrees, and efficiently expressing the modified sequence and the original sequence, the corresponding gene sequence was amplified by PCR amplification technology, and cloned into the expression vector pHY-WZX, Obtaining lactase expression plasmids pHY-Bgl-1, pHY-Bgl-2, pHY-Bgl-3, pHY-Bgl-4, pHY-Bgl-5, pHY-Bgl-6, pHY-Bgl-7, pHY-Bgl -8, pHY-Bgl-9, pHY-Bgl-10, pHY-Bgl-11, pHY-Bgl-12. The above-mentioned recombinant plasmids were trans...
Embodiment 2
[0092] Example 2: Genetic modification of expression host cells
[0093] Deletion of the aprE gene in Bacillus licheniformis CCTCC NO: M208236. With Bacillus licheniformis CCTCC NO: M208236 genomic DNA as a template, using apr-up1 (sequence 30) and apr-up2 (sequence 31) and primers apr-dn1 (sequence 32) and apr-dn2 (sequence 33) as primers, respectively The upper and lower homology arm fragments were amplified, and the sizes were 667bp and 495bp, respectively. After the PCR products of the correct size were obtained, they were purified by gel recovery, and overlapping PCR was performed using the DNA of the gel recovery product as a template to obtain a deletion mutant cassette △aprE with a size of ~1.2kb. After the mutant cassette was purified and digested with Xba I, it was cloned into plasmid pT2 tsThe Sma I and Xba I sites were transformed into Escherichia coli JM109 competent cells, cultured on LB plates containing 20 μg / mL kanamycin, and the correct deletion plasmid pT2...
PUM
| Property | Measurement | Unit |
|---|---|---|
| degree of polymerization | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com