Polyimide for gas separation prepared based on diaminotriptycene and derivatives thereof and preparation method thereof
A technology of diaminotriptycene and polyimide is applied in the field of polyimide for gas separation and its preparation, and can solve the problems of low gas permeability and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0032] Take 10.0g triptycene and 8.6g potassium nitrate into a 500mL round bottom flask, then add 200mL acetonitrile, then add 20mL trifluoroacetic anhydride, and react at room temperature for 12h. After the reaction, the obtained solution was added dropwise to deionized water to precipitate a solid, and the nitrated product was obtained by filtration;
[0033] Take 10 g of the above-mentioned nitrated product and add it to 300 mL of absolute ethanol, start cooling and reflux; then add 15 mL of 80% hydrazine hydrate and 1500 mg of target carbon catalyst, heat up to 120 ° C and reflux for 2 h;
[0034] After the reaction, use diatomaceous earth to filter and collect the filtrate, then add 200mL of deionized water to stir to produce a precipitate, place it in the refrigerator for recrystallization, filter, and dry in a vacuum oven at 80°C;
[0035]A mixture of petroleum ether and ethyl acetate with a volume ratio of 3:1 was used as a developer to adjust, and the obtained dried s...
Embodiment 2
[0038] Preparation of 2,7-diaminotriptycene-6FDA polyimide:
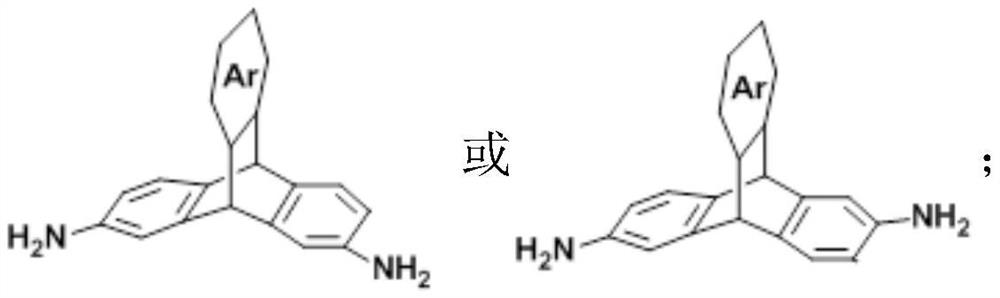
[0039] 284 mg of 2,7-diaminotriptylidene diamine prepared in Example 1 and 444.2 mg of hexafluorodianhydride were dissolved in 1.2 mL of m-cresol, and then a drop (0.05 mL) of isoquinoline was added to the solution as a catalyst, And raise the temperature to 160°C, and react for 2 hours; after the reaction, the obtained polymer is precipitated in ethanol solution, and m-cresol is removed by Soxhlet extraction to obtain 2,7 diaminotriptycene-6FDA polyimide.
[0040] 2,7 diaminotriptycene-6FDA polyimide was carried out by proton nuclear magnetic spectrum ( 1 H NMR, 400MHz, CDCl 3 ) test, the test results are: δ7.96(d, 2H, J=8.00Hz), 7.88(s, 2H), 7.80(d, 2H, J=7.60Hz), 7.53(d, 2H, J=8.00Hz ), 7.452 (s, 2H), 7.41 (m, 2H), 7.05 (m, 4H), 5.53 (d, 2H, J=12.8Hz). It can be seen that the synthetic results are consistent with the assumptions.
Embodiment 3
[0042] Preparation of 2,7-diaminotriptycene-6FDA:2,6-diaminotriptycene-6FD (1:1) polyimide:
[0043] 142 mg of 2,7-diaminotriptylidene diamine, 142 mg of 2,6-diaminotriptylidene diamine and 444.2 mg of hexafluorodianhydride prepared in Example 1 were dissolved in 1.2 mL of m-cresol, and then added to the solution A drop (0.05mL) of isoquinoline was used as a catalyst, and the temperature was raised to 160°C for 2 hours of reaction; after the reaction, the obtained polymer was precipitated in an ethanol solution, and m-cresol was removed by Soxhlet extraction to obtain 2,7- Diaminotriptycene-6FDA: 2,6-diaminotriptycene-6FD polyimide.
[0044] 2,7-diaminotriptycene-6FDA: 2,6-diaminotriptycene-6FD polyimide was carried out by proton nuclear magnetic spectrum ( 1 HNMR, 400MHz, CDCl 3 ) test, the test results are: δ7.98(d, 2H, J=5.2Hz), 7.88(s, 2H), 7.83(s, 2H), 7.53(d, 2H, J=7.6Hz), 7.43(d , 4H, J=14.6Hz), 7.04(m, 4H), 5.52(t, 2H). It can be seen that the synthetic results are...
PUM
| Property | Measurement | Unit |
|---|---|---|
| degree of polymerization | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com