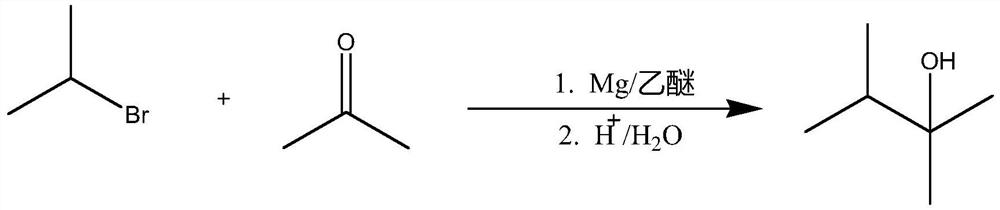Application method of Grignard reaction
A technology of Grignard reaction and application method, which is applied in the new application field of Grignard reaction, and can solve problems such as coupling side reactions, explosion, and flushing that are prone to occur
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0027]
[0028] Traditional two-step method:
[0029] 1, the preparation of isopropylmagnesium bromide:
[0030] In a 500mL four-necked bottle equipped with a thermometer, a condenser and a dropping funnel, add 10 grams of magnesium chips (0.42mol) and 150mL of anhydrous ether, respectively, and protect it with nitrogen, and add 50 grams (0.4mol) of bromine into the dropping funnel. A mixture of isopropane and 50 mL of anhydrous ether.
[0031] First add 3-4mL of the mixed solution into the four-neck flask, and the reaction starts to boil slightly after a few minutes. The reaction was intense at the beginning, and after it was relieved, the stirring was started, and the remaining mixed solution of ethyl bromide and ether was added dropwise, and the rate of addition was controlled so that the solution in the bottle was in a slightly boiling state. After the addition, heat and reflux in a warm water bath for 15 minutes, then cool down for later use.
[0032] 2. Preparation...
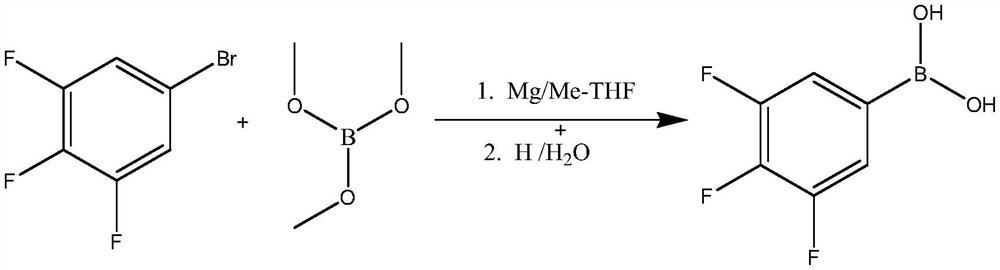
Embodiment 2
[0041]
[0042] Traditional two-step method:
[0043] Preparation of 3,4,5-trifluorophenylmagnesium bromide:
[0044] Add 42mmol (10.08g) of magnesium chips into a 500mL four-neck flask, add 2 grains of iodine, then add 200mL of anhydrous 2-methyltetrahydrofuran, heat the temperature to 50°C, and then let it cool down to 40°C naturally , and then drop 4-5mL of a mixed solution consisting of 84.4 grams (0.4mol) of 3,4,5-trifluorobromobenzene and 50mL of 2-methyltetrahydrofuran to initiate a Grignard reaction, and the temperature will rise rapidly after the reaction is initiated. Cool the temperature down to 10-15°C in an ice-water bath, then slowly add the remaining 3,4,5-trifluorobromobenzene mixed solution dropwise for about 2.0-3.0 hours, and react at room temperature for 8 hours after the dropwise addition.
[0045] Preparation of 3,4,5-trifluorophenylboronic acid:
[0046] Weigh 0.42mol (44g) of trimethyl borate, add it to a 1000mL four-neck flask, then add 200mL 2-meth...
Embodiment 3
[0051]
[0052] Traditional two-step method:
[0053] 1. Preparation of phenylmagnesium bromide:
[0054] Add 42mmol (10.08g) of magnesium chips into a 500mL four-necked flask, add 2 grains of iodine, and then add 200mL of anhydrous tetrahydrofuran, heat the temperature to 50°C, then let it cool down to 40°C naturally, and then add dropwise 4-5mL of a mixture consisting of 63 grams of bromobenzene (0.4mol) and 50mL of tetrahydrofuran will initiate a Grignard reaction, and the temperature will rise rapidly after the reaction is initiated. Use an ice-water bath to lower the temperature to 10-15°C, and then slowly add The remaining bromobenzene mixed solution, the dropwise addition time is about 2.0-3.0h, after the dropwise addition is completed, react at room temperature for 8h.
[0055] 2. Preparation of 2-phenyl-4,6-dichloro-1,3,5-triazine:
[0056] Weigh 0.42mol (78g) of cyanuric chloride, add it to a 1000mL four-neck flask, add 200mL tetrahydrofuran to dilute, put the f...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com