Preparation method of multi-stimulus synergistic response drug-release bone cement
A synergistic response, bone cement technology, used in pharmaceutical formulations, pharmaceutical science, drug delivery, etc., can solve the problem that the release bone cement cannot be accelerated and sustained drug release, etc., to prevent bone tumor recurrence, high drug loading, good The effect of the application foreground
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0059] A preparation method of a multi-stimuli synergistic response drug-releasing bone cement of the present invention, specifically:
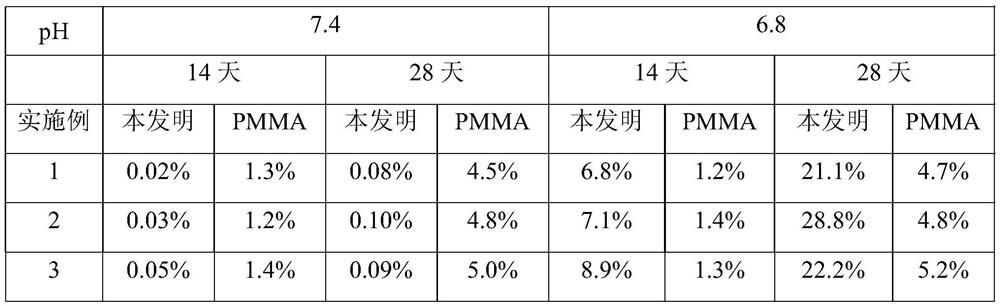
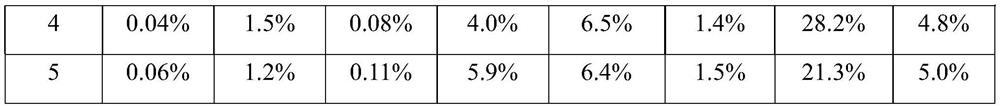
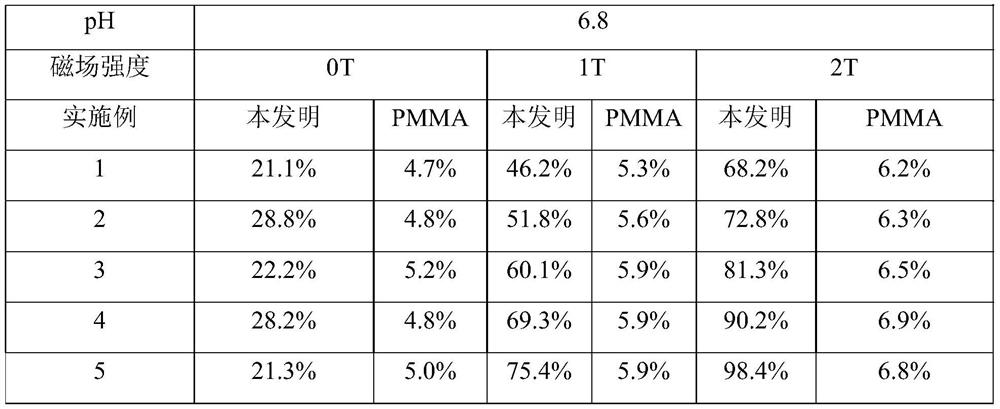
[0060]Step 1, mix MMA, DVB, drug and AIBN with a mass ratio of 1:0.1:0.11:0.01 evenly to obtain a polymerized monomer mixture; then add iron salt solution and heat to 50°C and stir to obtain a drug-loaded magnetic porous polymer The iron ions in the iron salt solution include ferric ions and ferrous ions, the molar ratio of ferric ions to ferrous ions is 10:5, and the volume ratio of MMA to ferric salt solution is 1:3 , the molar concentration of iron ions in the iron salt solution is 0.01mol / L; the drug is doxorubicin; the drug loading rate is 25%; the stirring time is 1h; the average particle size of the drug-loaded magnetic porous polymer particles is 1 μm;
[0061] Step 2, add the drug-loaded magnetic porous polymer particles into the mixed solution A, the solid-to-liquid ratio is 1:3g / ml; add the drug solution and stir for 6h, and precip...
Embodiment 2
[0072] A preparation method of a multi-stimuli synergistic response drug-releasing bone cement of the present invention, specifically:
[0073] Step 1, mix MMA, DVB, drug and AIBN with a mass ratio of 2:0.825:0.283:0.045 evenly, add iron salt solution and heat to 55°C and stir to obtain drug-loaded magnetic porous polymer particles; iron in iron salt solution The ions include ferric ions and ferrous ions, the molar ratio of ferric ions to ferrous ions is 1:0.625, the volume ratio of MMA to iron salt solution is 1:3.25, the molar concentration of iron ions in the iron salt solution is 0.133mol / L; the drug is DOX; the drug loading rate is 32.5%; the stirring time is 2h; the average particle size of the drug-loaded magnetic porous polymer particles is 2 μm;
[0074] Step 2, add the drug-loaded magnetic porous polymer particles to the mixed solution A, the solid-to-liquid ratio is 1:3.5g / ml; then add the drug solution and stir for 10.5h to precipitate CTAB and SiO 2 and n-hexane ...
Embodiment 3
[0085] A preparation method of a multi-stimuli synergistic response drug-releasing bone cement of the present invention, specifically:
[0086]Step 1, mix MMA, DVB, drug and AIBN with a mass ratio of 3:1.55:0.455:0.08 evenly, add iron salt solution and heat to 60°C and stir to obtain drug-loaded magnetic porous polymer particles; Iron ions include ferric ions and ferrous ions, the molar ratio of ferric ions to ferrous ions is 1:0.75, the volume ratio of MMA to iron salt solution is 1:3.5, and the molar ratio of iron ions in iron salt solution The concentration is 0.255mol / L; the drug is MTX; the drug loading rate is 40%; the stirring time is 3h; the average particle size of the drug-loaded magnetic porous polymer particles is 3 μm;
[0087] Step 2: Add the drug-loaded magnetic porous polymer particles into the mixed solution A, then add the drug solution and stir for 6-24 hours to precipitate CTAB and SiO 2 and n-hexane shell layer; after collecting microspheres, reflux in ac...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com