Lithium ion conductive composite material for all solid-state lithium battery, and solid polymer electrolyte and all solid-state lithium battery including the same
A lithium ion and lithium battery technology, applied in the field of lithium ion conductive composition, can solve the problems of adverse effects on the safety of lithium ion batteries, low lithium ion conductivity, reduced battery performance, etc., and achieve high room temperature and high temperature lithium ion conductivity. , the effect of high coulombic efficiency, high room temperature and high temperature discharge gram capacitance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0033] The lithium ion conductive composition and solid polymer electrolyte of Example 1 of the present invention are prepared by a method comprising the following steps:
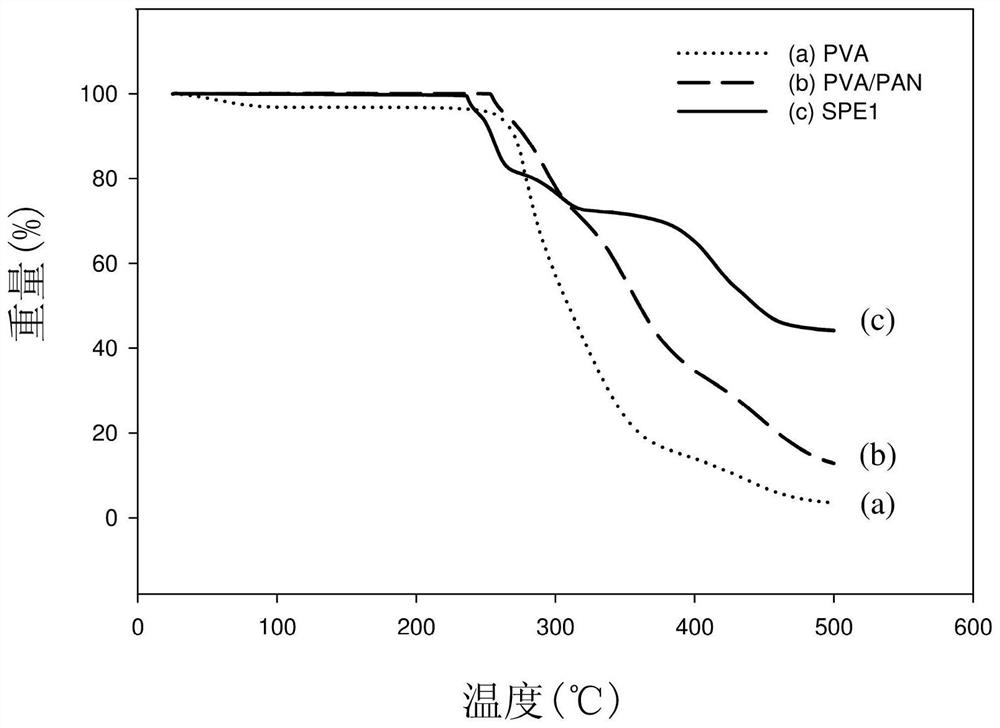
[0034] PVA (M w =8.9×10 5 , purchased from Sigma-Aldrich) and PAN (M w =1.5×10 5 , purchased from Sigma-Aldrich) mixed in a weight ratio of 92.5:7.5 to obtain a polymer blend (PVA / PAN), followed by lithium bis(trifluoromethanesulfonyl)imide (LiTFSI, purchased from Sigma- Aldrich) was mixed with PVA / PAN and dissolved in dimethyl sulfoxide (DMSO, purchased from Sigma-Aldrich). Then, lithium aluminum titanium phosphate (LATP) and succinonitrile (SN, purchased from Sigma-Aldrich) were added to the above DMSO solution under stirring, wherein the weight ratio of PVA / PAN, LiTFSI, LATP, and SN was 4: 4:2:0.4, heated to 80° C. and kept under stirring for 24 hours to obtain a solution (ie, the lithium ion conductive composition of Example 1).
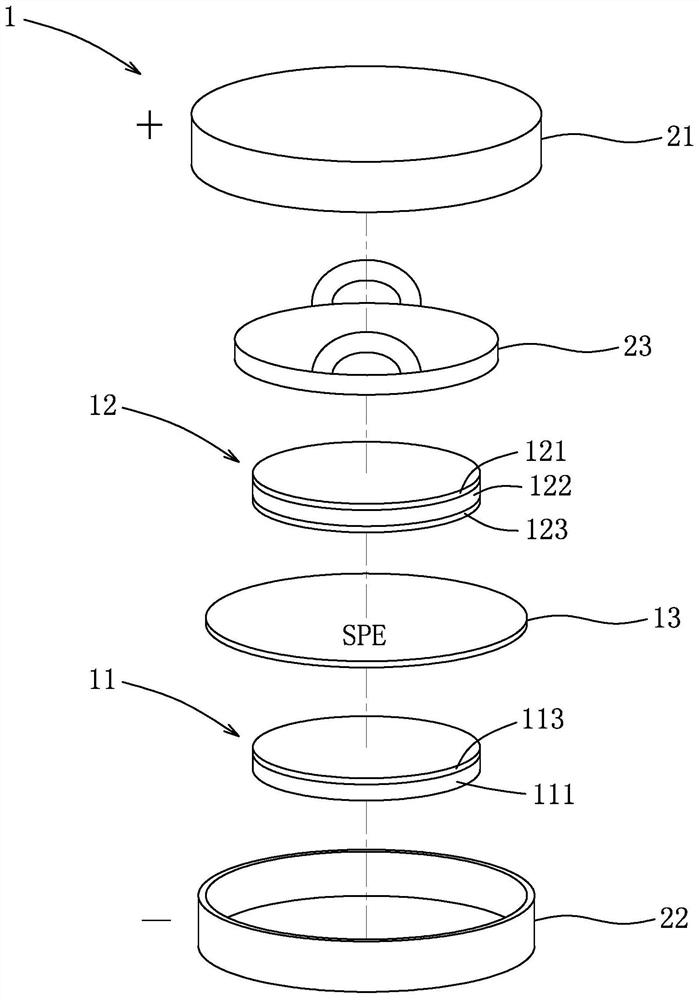
[0035] Subsequently, the uniformly stirred solution was coated on a ...
Embodiment 2
[0038] The lithium ion conductive composition and solid polymer electrolyte of Example 2 of the present invention are prepared by a method comprising the following steps:
[0039] (1) LiNO 3 (purchased from Alfa Aesar), Al(NO 3 ) 3 9H 2 O (purchased from Alfa Aesar) and La (NO 3 ) 3 ·6H 2 O (purchased from Alfa Aesar) was mixed and stirred at a molar ratio of 6.25:0.25:3 for 30 min to be dissolved in deionized water. (2) In addition, zirconium tetrapropoxide solution (70wt% propanol solution, purchased from Sigma-Aldrich) was dissolved in isopropanol containing 15vol% acetic acid, wherein the ratio of Zr was based on the above (1) Depending on the amount of La used, the molar ratio of La and Zr is 3:2, and an excess of LiNO is added 3 to a concentration of 15wt% to compensate for subsequent loss of lithium during high-temperature calcination.
[0040] The above two solutions (1) and (2) were mixed and stirred for 30 min to form an Al-doped LLZO solution, and then the g...
Embodiment 3
[0044] The lithium ion conductive composition and solid polymer electrolyte of Example 3 of the present invention are prepared by a method comprising the following steps:
[0045] PVA, Al-LLZO and LiTFSI prepared in Example 2 above were mixed and dissolved in DMSO to obtain the first mixture (PVA / Al-LLZO / LiTFSI). Separately, PAN and SN were mixed and dissolved in DMSO to obtain a second mixture (PAN / SN).
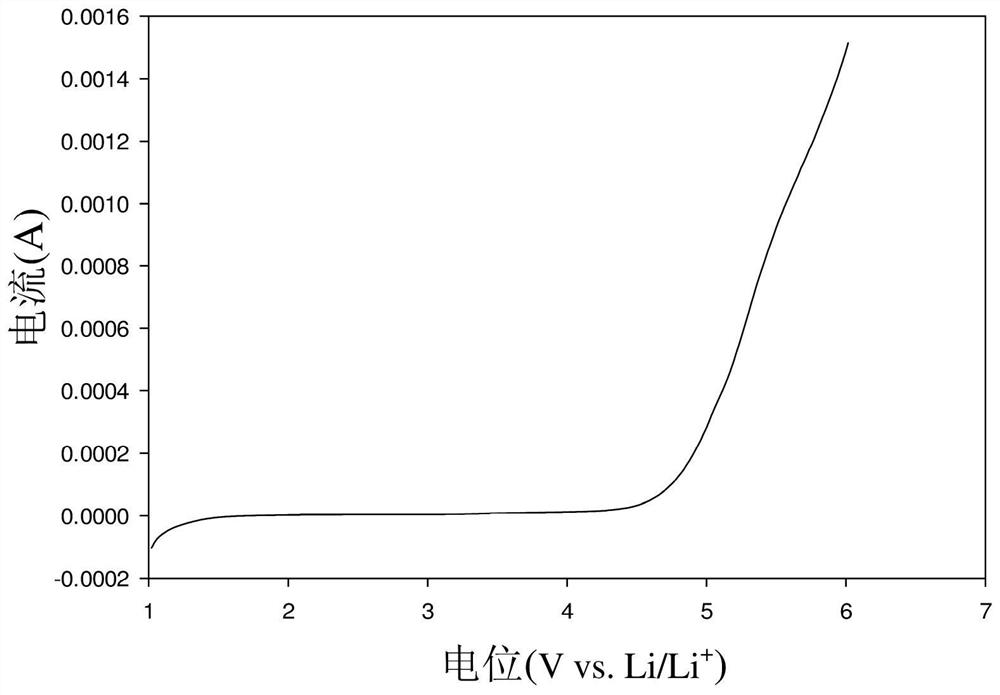
[0046] Mix the above first mixture and the second mixture, the weight ratio of PVA and PAN is 92.5:7.5, the weight ratio of the sum of PVA and PAN, LiTFSI, Al-LLZO, SN is 4:4:2:0.4, heat to 80 ℃ and maintained under stirring for 24 hours, and ball milled at a speed of 400 rpm for 2 hours to obtain a uniform solution (ie, the lithium ion conductive composition of Example 3). Then the solution was coated on glass, dried at room temperature (25° C.) for 24 hours, and vacuum-dried at 70° C. for 72 hours to obtain the solid polymer electrolyte membrane SPE3 of Example 3. Finall...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Li-ion conductivity | aaaaa | aaaaa |
| Diameter | aaaaa | aaaaa |
| Thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com