Application of viologen coordination compound crystal as multifunctional color-changing material
A technology of coordination compounds and color-changing materials, applied in the application field of viologen coordination compound crystals as multi-functional color-changing materials, can solve the bottleneck period, the development and application limitations of such materials, isomerization and other problems, and achieve the speed of color change. quick effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0035] Example 1 Multifunctional viologen complex crystal (1)
[0036] 1. Preparation of viologen compound 1,1-bis(3-carboxyphenyl)-4,4-bipyridine dichloride (H 2 bcbpy 2Cl)
[0037] 4,4'-bipyridine (2g, 12.8mmol) and 3-chloromethylbenzoic acid (6.56g, 38.4mmol) were added into a 50mL round bottom flask filled with 13mL of N,N-dimethylformamide, in N 2 Under the protection of air, heat and reflux at 120°C for 8h, then cool to 25°C and filter to obtain a yellow precipitate, wash with hot DMF solution three times, then wash with ethanol three times, and dry in vacuum at 70°C for 12h . After recrystallization with acetone and deionized aqueous solution at a volume ratio of 1:1, H 2 bcbpy·2Cl viologen compound. The yield was 93%, elemental analysis C 26 h 22 o 4 N 2 Cl 2 (%): Theoretical value: C, 62.80; H, 4.42; N, 5.64%. Experimental values: C, 62.75; H, 4.58; N, 5.59%.
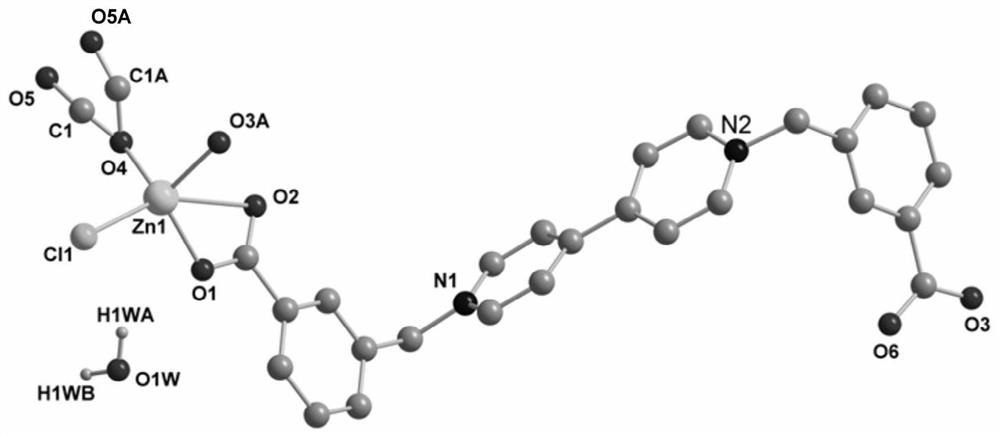
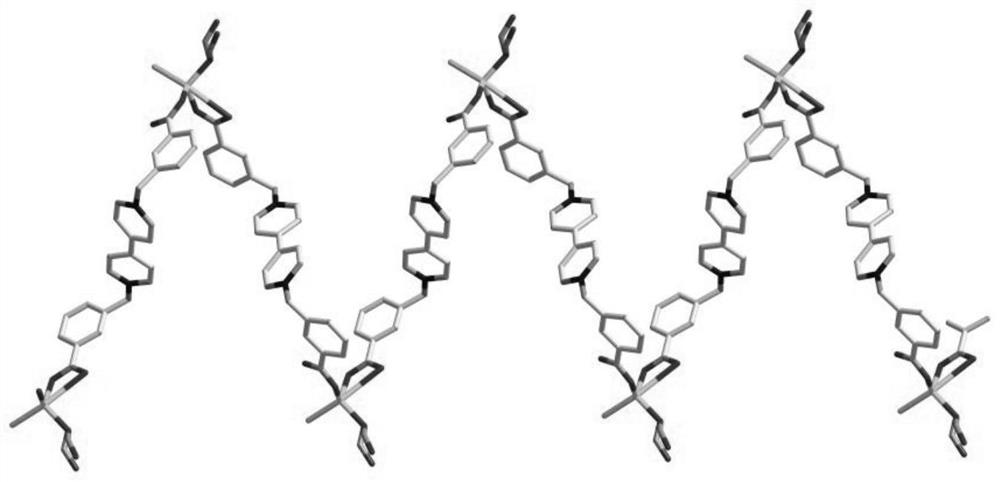
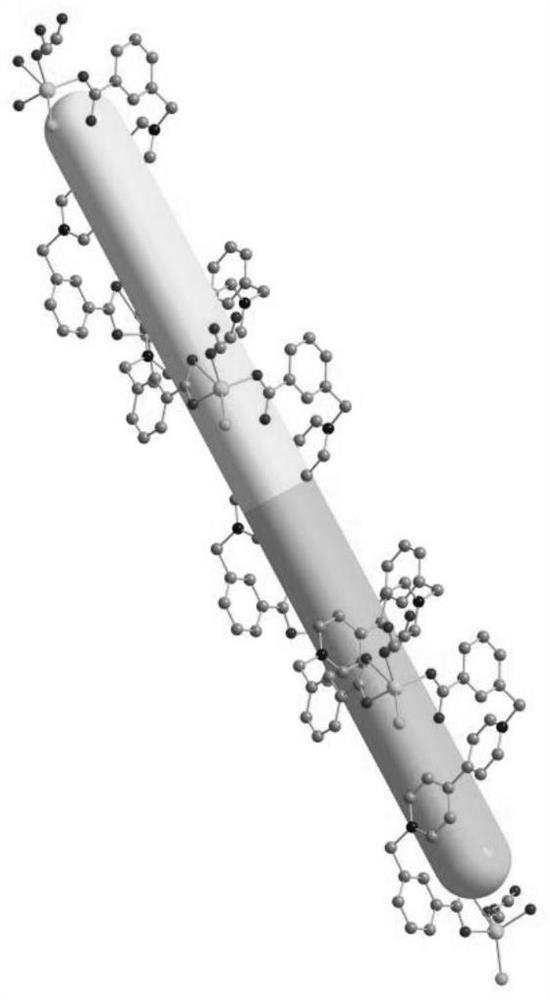
[0038] 2. Preparation of multifunctional viologen complex crystal [Zn(Ma)(bcbpy)Cl]·2H 2 o
[...
Embodiment 2
[0054] Example 2 Multifunctional Viologen Complex Crystal (2)
[0055] Zn(NO 3 ) 2 ·6H 2 O (0.15mmol, 45mg) and H 2 bcbpy·2Cl (0.05mmol, 24.8mg) was dissolved in a solution of 3ml deionized water and 3ml N,N-dimethylformamide, and dissolved by stirring, and the dissolved solution was placed in a 20ml polytetrafluoroethylene In a lined reaction kettle, the synthesis was carried out by solvothermal method. After the temperature was kept at 90°C for 2 days, it was cooled to room temperature, washed with deionized water, and then light yellow needle-like crystals were obtained, which was the target compound. The yield was 85%. Elemental Analysis C 27 h 25 o 8.5 N 2 ClZn (%): Theoretical: C, 43.71; H, 3.39; N, 3.46%. Experimental values: C, 43.11; H, 3.45; N, 3.33%.
Embodiment 3
[0056] Example 3 Multifunctional Viologen Complex Crystal (3)
[0057] Zn(NO 3 ) 2 ·6H 2 O (0.3mmol, 90mg) and H 2 bcbpy·2Cl (0.1mmol, 49.7mg) was dissolved in a solution of 2ml deionized water and 4ml N,N-dimethylformamide, and dissolved by stirring, and the dissolved solution was placed in a 20ml polytetrafluoroethylene In a lined reaction kettle, the synthesis was carried out by solvothermal method. After constant temperature at 85°C for 3 days, it was cooled to room temperature, washed with deionized water, and light yellow needle-like crystals were obtained, which was the target compound. The yield was 86%. Elemental Analysis C 27 h 25 o 8.5 N 2 ClZn (%): Theoretical: C, 43.71; H, 3.39; N, 3.46%. Experimental values: C, 43.12; H, 3.47; N, 3.36%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com