A Visible Light-Driven Boron Concentrated Photochromic Material and Its Preparation and Application
A photochromic material and visible light technology, applied in the direction of color-changing fluorescent materials, chemical instruments and methods, compounds containing elements of group 3/13 of the periodic table, etc., can solve problems such as limiting the development of boron-heterocondensed ring systems, and achieve excellent results The effect of quantum yield and broad application prospect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0068]
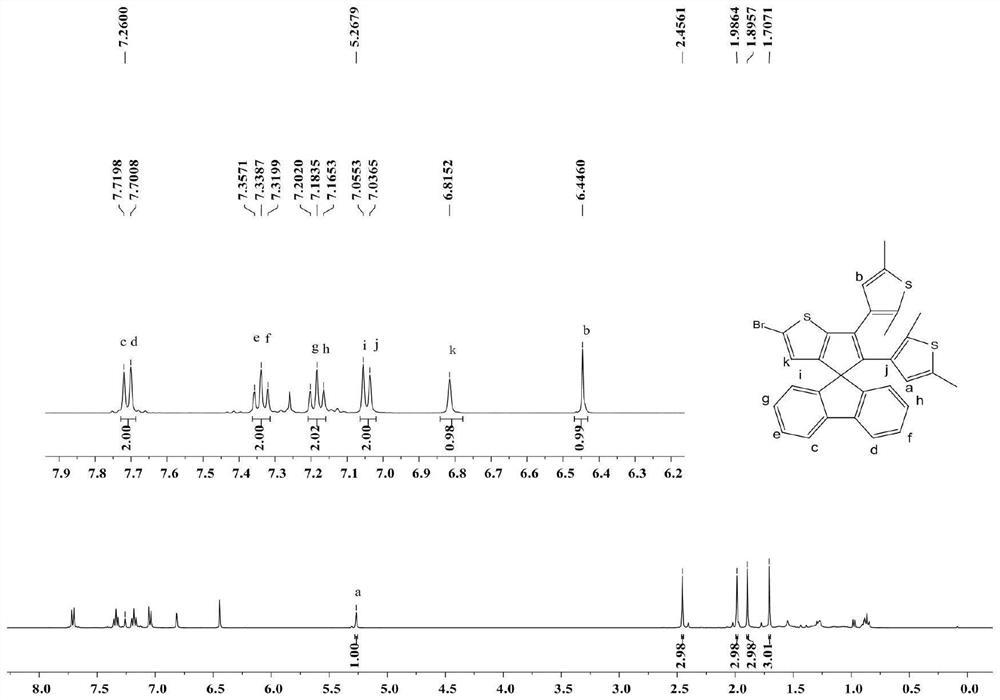
[0069] Compound A (200 mg, 0.4 mmol) and anhydrous dichloromethane (50 mL) were added to a dry 100 mL one-neck flask, and after fully dissolving, it was cooled to 0°C. In the dark, N-bromosuccinimide (79.5 mg, 0.4 mmol) was added portionwise. The reaction was performed at room temperature in the dark for 4 h, and the reaction process was monitored by spot plate. After the reaction was completed, 2 mL of water was added to quench the reaction. It was then extracted with 50 mL of saturated brine and 50 mL of dichloromethane. Anhydrous Na for organic phase 2 SO 4 After drying, the solvent was removed by suction filtration. The residue was purified by flash column chromatography on silica gel (petroleum ether) to give 227 mg of yellow compound B in 98% yield.
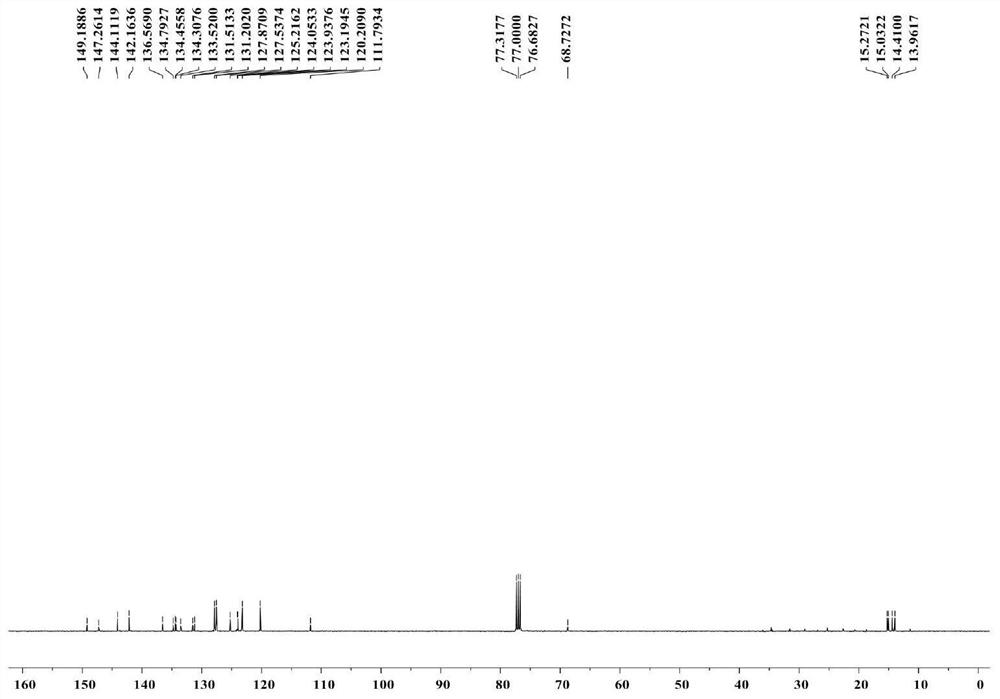
[0070] The structure of the compound of formula B was confirmed by means of hydrogen NMR and carbon spectroscopy.
[0071] 1 H NMR (400MHz, CDCl 3 ): δ7.71(d,J=7.6Hz,2H),7.34(t,J=7.4Hz,2H),7.18(t,J=7.3Hz...
Embodiment 2
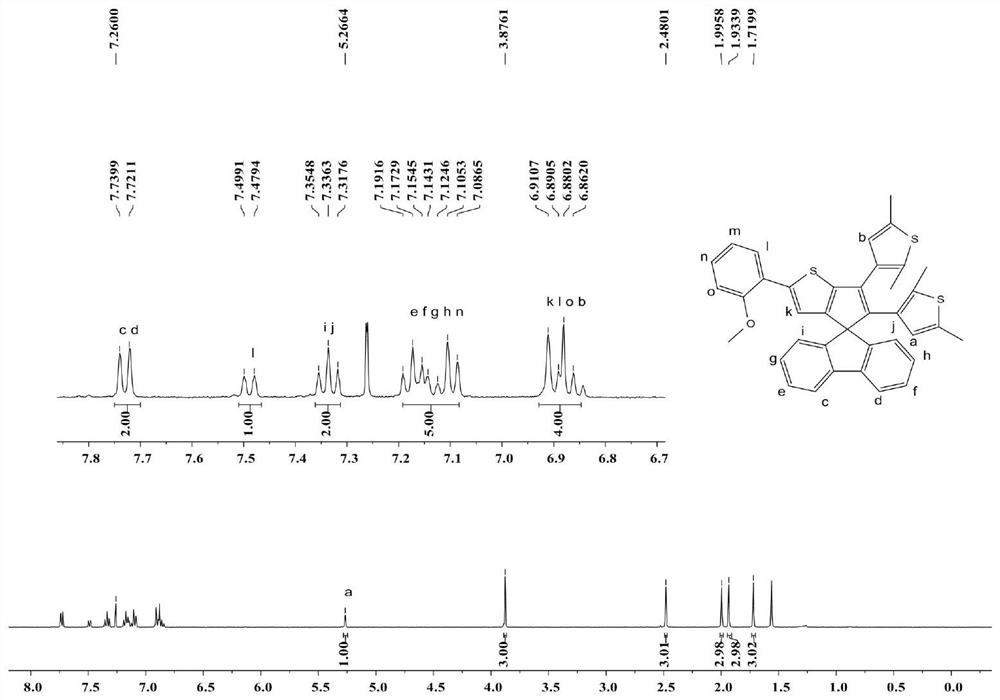
[0084] At room temperature, we have a 5×10 -5 The tetrahydrofuran solution of mol / L compound D was tested by ultraviolet absorption spectrum, and the results were as follows Figure 7 (A). Compound D at 382nm (ε=2.64×10 4 L mol -1 cm -1) appeared at a strong absorption peak, the absorption end extended to about 445nm, which can be excited by visible light. After exposure to visible light at ~400 nm, compound D undergoes a ring-closure reaction, and at 487 nm (ε=5.66×10 3 Lmol -1 cm -1 ) a new absorption band appears. With light exposure time, photosteady state was reached after 45 s. This process is also accompanied by a color change of the compound, and the solution turns from colorless to yellow. Under visible light (λ>450nm) illumination, the yellow solution returned to the original colorless solution. like Figure 7 As shown in (B), the tetrahydrofuran solution of compound D can be excited by alternating short-wave and long-wave, and can reciprocate more than te...
Embodiment 4
[0086] Fluorescence properties of compound D in tetrahydrofuran solution. The result is as Figure 8 As shown, an obvious fluorescence emission peak appeared at 446 nm, and with the prolongation of illumination, the fluorescence intensity there gradually weakened, and the photo-steady state was reached after 45 s. This may be due to the overlapping of the ultraviolet absorption spectrum and the fluorescence emission spectrum of the ring-closed isomer D, and there is an energy transfer (FRET) process, resulting in fluorescence quenching. The isolated pure ring-opening isomer D, the fluorescence quantum yield of its tetrahydrofuran solution is Φ F = 2.14% (with 0.5M quinoline sulfate as reference).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com