2, 3-dihydro-3-O-acyl-5-hydroxy-6-methyl-4H-pyran-4-one, and preparation method and application thereof
A technology of dihydroxy and methyl, which is applied in the field of synthesis of tobacco flavors, can solve the problems of perishable damage, failure to make cigarettes, application, poor stability, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
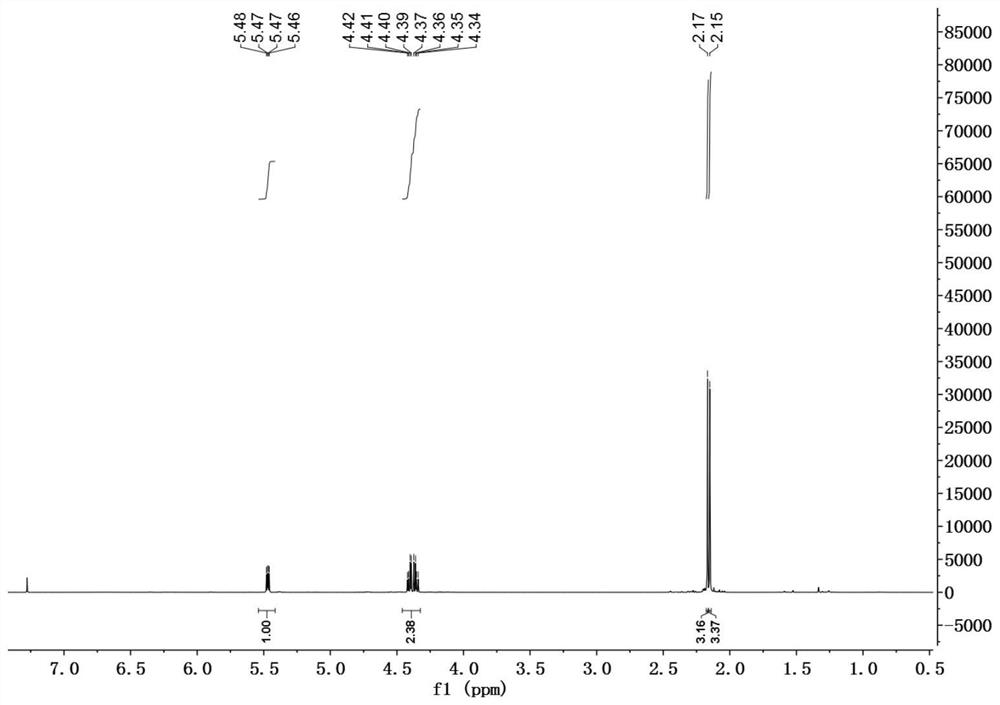
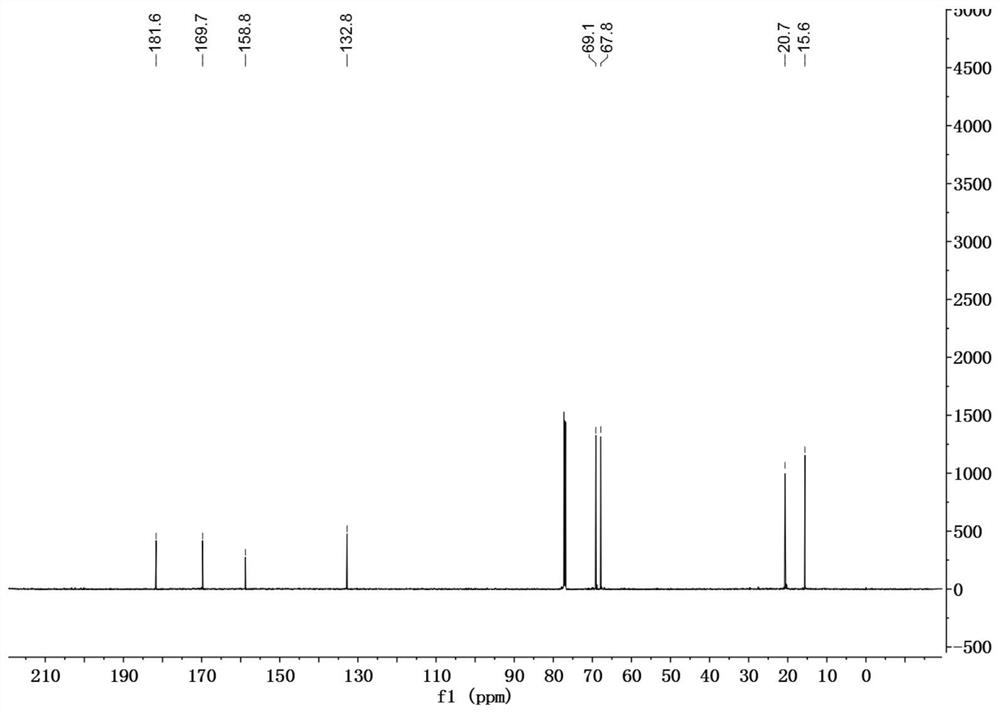
[0030] In a 50mL reaction flask, DDMP (1.44g, 10mmol) was dissolved in 20mL of anhydrous dichloromethane, and the catalyst 4-dimethylaminopyridine (DMAP) (0.06g, 0.5mmol), triethylamine (1.2g, 11mmol) were added successively ), BOC 2 O (2.3g, 10.5mmol), reacted at room temperature for 3h, evaporated the solvent, and the residue was subjected to silica gel column chromatography, eluting with petroleum ether / ethyl acetate=3 / 1 to obtain 2.0g of DDMP-5-BoC compound, the yield 95%.
[0031] DDMP-5-BoC compound (0.68g, 2.8mmol) was dissolved in 20mL of anhydrous dichloromethane, cooled to 0°C, triethylamine (0.36g, 3.6mmol), acetyl chloride (0.24g, 3.1mmol) were added successively, After adding, it was raised to room temperature for 4h, and the reaction solution was successively washed with saturated NaHCO 3 , washed with saturated NaCl, and the organic phase was washed with anhydrous NaCl 2 SO 4 After drying, 0.76 g of DDMP-5-BoC-3-acetate compound was obtained with a yield of ...
Embodiment 2
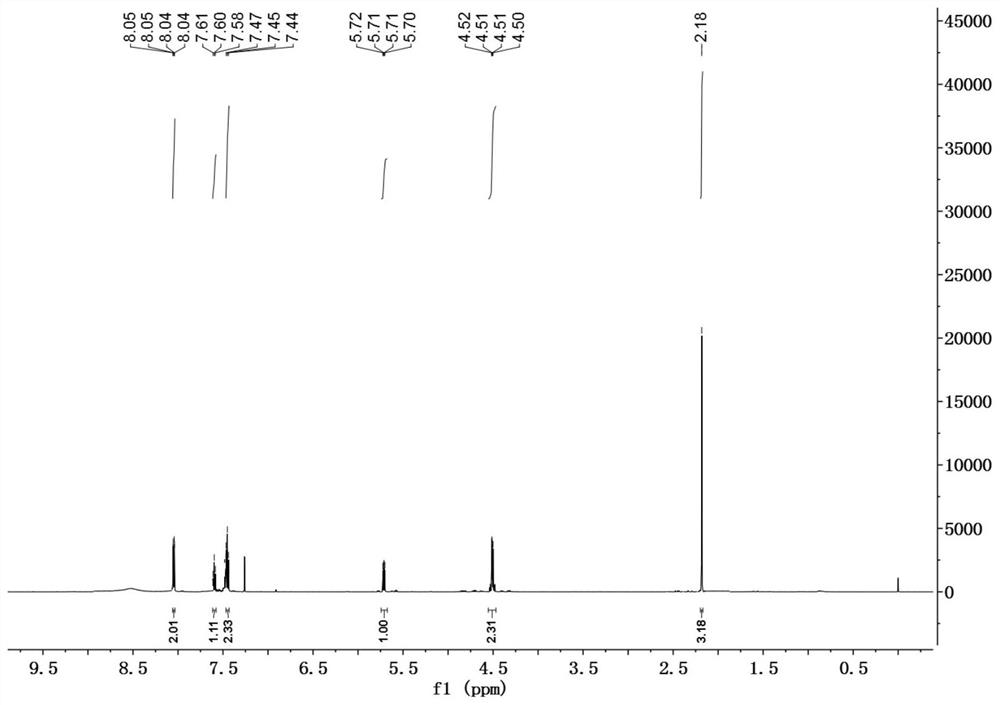
[0034] In a 50mL reaction flask, successively add DDMP (1.44g, 10mmol) dissolved in 20mL of anhydrous methanol, successively add triethylamine (1.2g, 11mmol), DMAP (0.06g, 0.5mmol), BOC 2 O (2.3g, 10.5mmol), reacted at room temperature for 3h, evaporated the solvent, and the residue was subjected to silica gel column chromatography, eluting with petroleum ether / ethyl acetate=3 / 1 to obtain 2.0g of DDMP-5-BoC compound, the yield 95%.
[0035] DDMP-5-BoC compound (0.6g, 2.8mmol) was dissolved in 20mL of anhydrous dichloromethane, cooled to 0°C, and triethylamine (0.36g, 3.6mmol) and phenylacetyl chloride (0.48g, 3.1mmol) were added successively , Add Bi rose to room temperature for 6h. The reaction solution was sequentially washed with saturated NaHCO 3 , washed with saturated NaCl, and the organic phase was washed with anhydrous NaCl 2 SO 4 After drying, 0.88 g of DDMP-5-BoC-3-benzoate compound was obtained with a yield of 90%.
[0036] DDMP-5-BoC-3-benzoate compound (0.35g...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com