Preparation method of elagolix sodium and intermediate thereof
A technology for elagolix sodium and intermediates, which is applied in the field of pharmaceutical synthesis and preparation, can solve the problems of unfavorable large-scale industrial production, complicated post-treatment of environmental pollution, and difficulty in impurity removal and purification, and achieves easy scale-up, low cost, and strong The effect of cost competitiveness
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
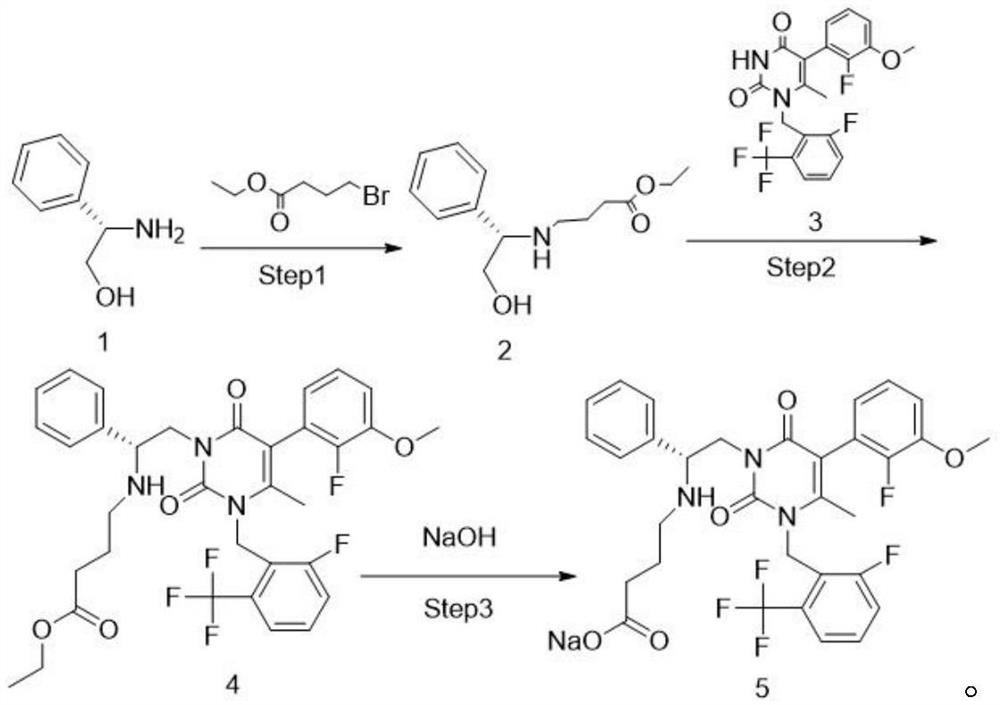
[0041] A preparation method of elagolix sodium and intermediates thereof, comprising the following preparation steps:
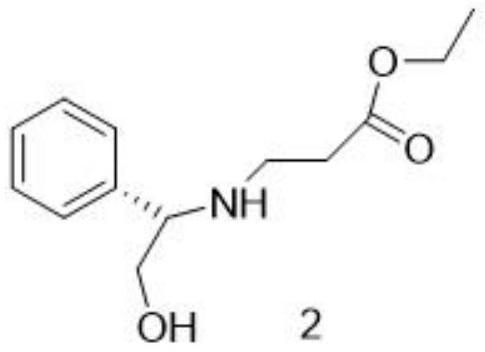
[0042] (1) Substitution reaction: Dissolve D-phenylglycinol 1 in tetrahydrofuran, add ethyl 4-bromobutyrate under stirring, and stir the reaction. If there is no raw material point detected by TLC, the reaction is stopped, and the reaction solution is layered with brine. The organic phase was concentrated and stripped with tetrahydrofuran to obtain the reaction solution of compound 2
[0043] The reaction formula is:
[0044]
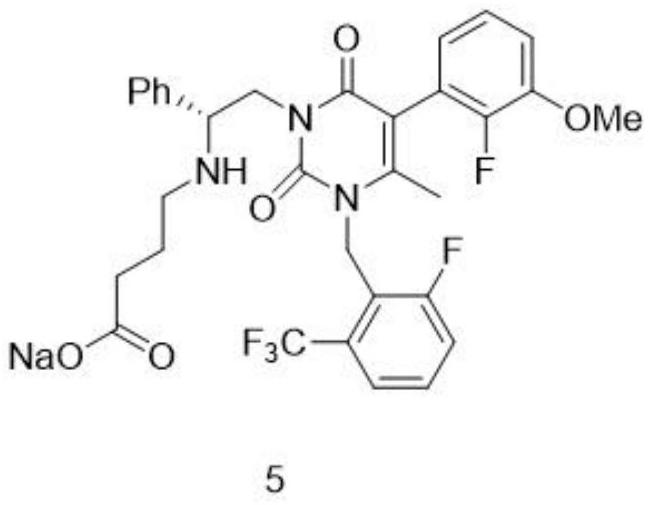
[0045] (2) Condensation reaction: add NMP and compound 3 in compound 2, stir reaction, HPLC detects purity: compound 1≤0.5% (wavelength 210nm), stop reaction, drop to room temperature, add IPAC and water washing in reaction liquid, separate Organic phase, the organic phase has passed through H 3 PO 4 ,IPAC,K 2 CO3 Extraction, and finally through concentration, the concentrated product is stripped with EtOH to obtain the EtOH rea...
Embodiment 1
[0061] Step (1) compound 1 and ethyl 4-bromobutyrate react to generate compound 2
[0062] The synthetic route equation is as follows:
[0063]
[0064] In a reaction vessel, the compound 1 (1g, 1eq) was dissolved in tetrahydrofuran (4ml, 4V), and ethyl 4-bromobutyrate (1.5g, 1.05eq) was added thereto, and the reaction was stirred at 50°C for 3h; After the reaction, the reaction solution was washed with saturated brine, and the organic phase was concentrated and entrained with tetrahydrofuran twice. 1.65 g of compound 1 was obtained with a yield of 90%.
[0065] The NMR data of compound 3 are as follows:
[0066] 1H-NMR (400MHz, Chloroform-d) δ10.63(s, 1H), 4.90(t, J=1.1Hz, 1H), 2.34(d, J=1.0Hz, 3H).
[0067] Step (2) compound 2 and compound 3 react to generate compound 4
[0068]
[0069] In the reaction vessel, the compound 2 (1g, 1eq) was dissolved in NMP (2ml, 1X), and compound 2 (1.3g, 0.6eq) was added thereto, and the reaction was stirred at 50°C for 20h; after...
Embodiment 2
[0080] Step (1) In a reaction vessel, the compound 1 (1g, 1eq) was dissolved in 2ml of dichloromethane (2ml, 2X), and ethyl 4-bromobutyrate (1.35g, 0.95eq) was added thereto. The reaction was stirred at 20° C. for 3 h; after the reaction, the reaction solution was washed with saturated brine, and the organic phase was concentrated and entrained with tetrahydrofuran twice. Finally, 10.77 g of compound was obtained with a yield of 42%.
[0081] Step (2) In a reaction vessel, dissolve Compound 2 (1g, 1eq) in dichloromethane (10ml, 10X), add Compound 2 (1.5g, 0.7eq) to it, and stir the reaction at 40°C for 20h ; After the reaction, the reaction solution was concentrated and washed with ethyl acetate and water, and the organic phase was separated; the organic phase was successively passed through 10% H 3 PO 4 , ethyl acetate, 20% K 2 CO 3 After extraction and concentration, the concentrated product was stripped with EtOH to obtain a relatively pure EtOH reaction solution of com...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com