Enzyme-linked immunoassay method for quantitatively detecting content of anti-human interleukin 17 monoclonal antibody in serum
A monoclonal antibody and antibody technology, applied in the biological field, can solve the problems of low chromatography sensitivity, unstable recovery rate, cumbersome test process, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0150] Example 1 Preparation and Purification of Biotin-labeled Secondary Antibody
[0151] Anti-human interleukin-17 monoclonal antibody (F(ab) 2 ) as an antigen for rabbit immunization, and through B cell cloning technology to screen out the monoclonal antibody that can bind to the monoclonal antibody, does not block the binding of the anti-human interleukin-17 monoclonal antibody to its target human interleukin-17, and does not cross-react with other human IgG of the same type non-neutralizing rabbit monoclonal antibody.
[0152] After obtaining the above-mentioned anti-antibody light / heavy chain gene fragment and sequence information, first, design and synthesize the 3' end primer containing the Avi-Tag gene sequence; then use the PCR method to amplify and obtain the 3' end containing Avi-Tag sequence chain gene; by one-step cloning method, the heavy chain gene containing Avi-Tag was inserted into the expression plasmid to construct the heavy chain expression plasmid cont...
Embodiment 2
[0153] Embodiment 2 Carry out performance appraisal to the monoclonal antibody made by embodiment 1
[0154] 1. Identification of binding activity
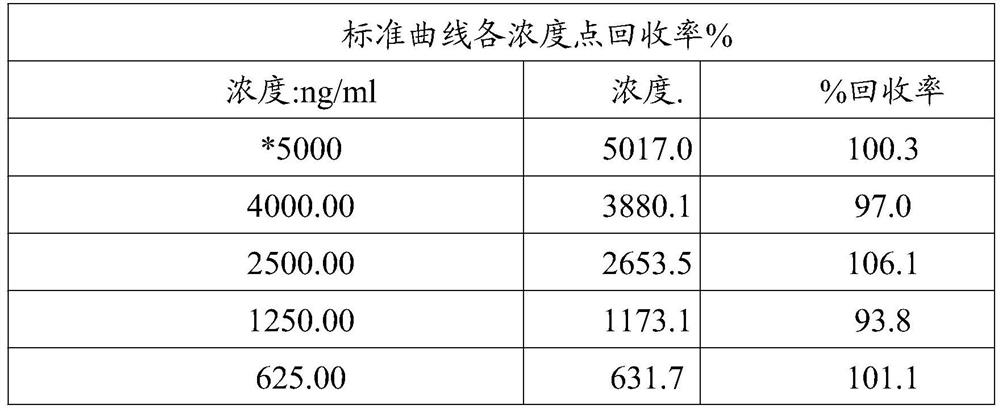
[0155] Use anti-human interleukin-17 monoclonal antibody and human IgG as antigens to coat the enzyme-linked plate; make the serially diluted monoclonal antibody in Example 1 react with the coated antigen; sequentially add chromogenic antibody; substrate chromogenic solution; terminate solution; for binding activity experiments. The results showed that the monoclonal antibody had good binding activity to the anti-human interleukin-17 monoclonal antibody, and did not cross-react with isotype IgG.
[0156] 2. Identification of neutralizing activity
[0157] Use human interleukin-17 as an antigen to coat the enzyme-linked plate; add the incubation of the quantitative anti-human interleukin-17 monoclonal antibody and the serially diluted monoclonal antibody in Example 1 to react with the coated antigen; add the chromogenic antibody ...
Embodiment 3
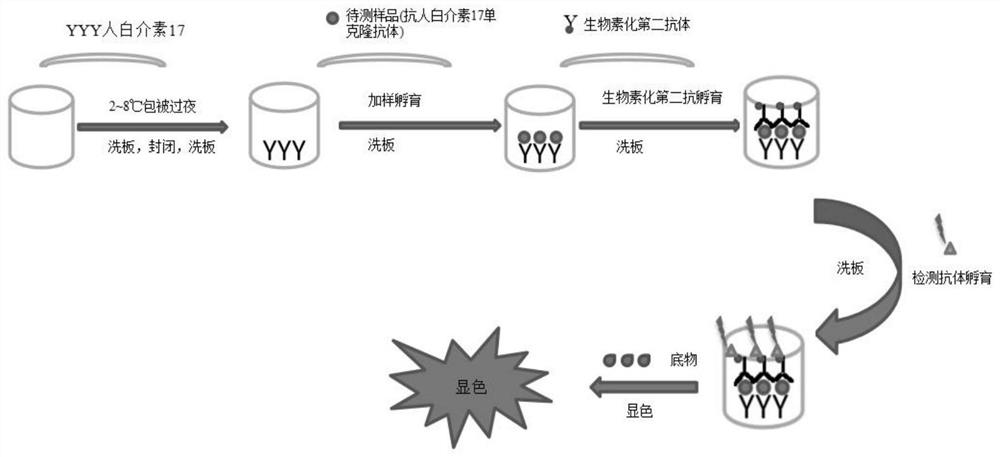
[0160] Example 3 Using the "Secondary Antibody" Prepared in Example 1 to Construct an Elisa Kit
[0161] The antigen is interleukin-17 and is immobilized on the enzyme-linked plate; anti-human interleukin-17 monoclonal antibody can bind to the coated antigen; the "secondary antibody" prepared in Example 1 is used as the second antibody; horseradish peroxidase-labeled Streptavidin was used as a chromogenic antibody.
[0162] experiment procedure:
[0163] Coating: human interleukin-17 was diluted to 1 μg / ml with 1×PBS, 100 μl / well, and coated overnight at 2-8°C;
[0164] Blocking: 200 μl / well blocking solution, blocking at room temperature for 2 hours;
[0165] Adding samples: Dilute the MRD 20 times with the diluent before adding the standard and quality control samples; 100 μl / well, incubate at room temperature for 2 hours;
[0166] Add secondary antibody: add the "secondary antibody" prepared in Example 1 to diluent to 10ng / ml, 100μl / well, incubate at room temperature for...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com