Novel conductive polymer and preparation method and application thereof
A conductive polymer and polymer technology, applied in the field of new conductive polymers and their preparation, can solve problems such as difficulty in preparation, difficult synthesis routes, and poor solubility, and achieve solubility optimization, strong promotion and application value, and improved solubility Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
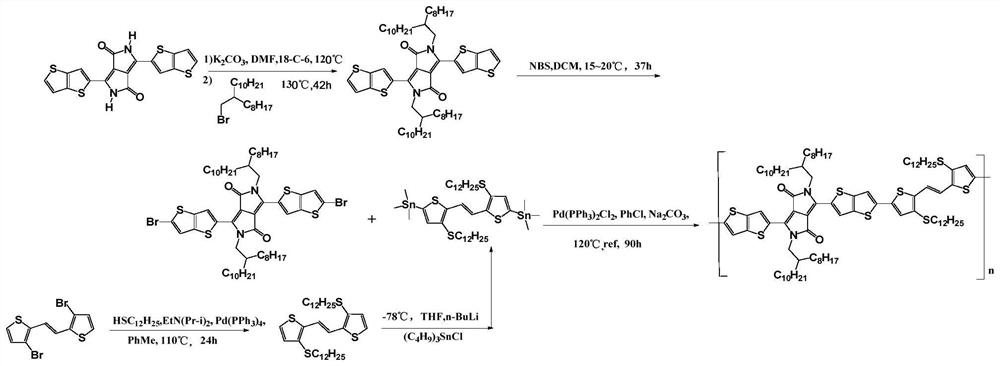
[0039] On the other hand, the embodiment of the present application also provides a preparation method of a novel conductive polymer, comprising the following steps:
[0040] 3,6-bis(bromodithiophen-2-yl)-2,5-diRpyrrole[3,4-c]pyrrole-1,4-dione and bistanno(E)-1, 2-bis(3-R'-thiophen-2-yl)ethylene is added to the organic solvent to obtain a mixed solution;
[0041] A catalyst is added to the mixed solution, and the mixed solution is subjected to a Stille coupling reaction at a set reaction temperature and a set reaction time to obtain the polymer.
[0042] In the examples of the present application, the 3,6-bis(bromodithiophen-2-yl)-2,5-diR pyrrole[3,4-c]pyrrole-1,4-dione has the following formula II structure:
[0043]
[0044]The bistin generation (E)-1,2-bis(3-R'-thiophen-2-yl)ethylene has the following structure of formula III:
[0045]
[0046] Wherein, the R' is a sulfanyl group and / or an oxyalkyl group, and the number of carbon atoms of the R' is 6-16.
[0047] ...
Embodiment 1
[0056] Example 1: Synthesis of (E)-1,2-two (3-bromo-2-thiophene) ethylene
[0057] See figure 1 , figure 1 It is a synthetic route diagram of (E)-1,2-bis(3-bromo-2-thiophene) ethylene (TVT) in the embodiment of the application, and the E)-1,2-bis(3 The specific preparation process of -bromo-2-thiophene) base ethylene is as follows:
[0058] At 0°C, under anhydrous and oxygen-free conditions, add 3-bromothiophene (100mmol), dry THF (100ml) and strong base LDA (120mmol) into the flask, react for 1h, then add dry DMF (120mmol), and react 0.5h, after the reaction was finished, it was lowered to room temperature, overnight, after-treatment to obtain a brown liquid, which was the intermediate product 4-bromothiophene-2-carbaldehyde, and the yield was 52%; the above-mentioned brown liquid was detected by nuclear magnetic resonance: 1H NMR (300MHz , d-DMSO ppm): δ=7.28 (s, H), 7.37 (s, H), 9.41 (s, H).
[0059] TiCl4 (20mmol) was added dropwise to a flask equipped with dry THF (30...
Embodiment 2
[0060] Example 2: Synthesis of Polymer P1
[0061] A preparation method of a novel conductive polymer, the polymer P1 has the following general formula:
[0062]
[0063] The method comprises the following steps: 3,6-bis(dithiophen-2-yl)-2,5-dihydropyrrole[3,4-c]pyrrole-1,4-dione (BT-DPP)( 200mmol), K2CO3 (8.0mmol), DMF (1000ml) were added to a 5L three-necked flask, replaced with nitrogen twice, the temperature was raised to 120°C, the phase transfer catalyst 18-crown-6 was added, and 9-(bromomethylene) was added dropwise Nonadecane (600mmol), after dropping, heated up to 130°C, reacted for 48h, after the end, cooled down to room temperature, extracted, separated, dried to obtain 2,5-bis(2-octyldodecyl)- BT-DPP. Add the obtained 2,5-bis(2-octyldodecyl)-BT-DPP (80mmol), NBS (80mmol), and DCM (dry 400ml) into a 700mL three-necked flask, and replace it with nitrogen twice. Temperature 15℃~20℃, react for 37h, after the reaction, hydrolyze, wash with water, and dry to obtain...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com