Chewable tablet containing herba cistanche and preparation method of chewable tablet
A technology of chewable tablets and Cistanche, which is applied to the functions of food ingredients, food ingredients containing natural extracts, food ingredients containing polysaccharides/gum, etc., can solve the problems of difficult product quality assurance, high water content of Cistanche, and loss of active ingredients. To achieve the effect of short production cycle, increase added value, and improve color
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0040] The present invention also provides a preparation method for the above-mentioned chewable tablet containing Cistanche deserticola, comprising the following steps: mixing the raw and auxiliary materials except the lubricant, adding a wetting agent to granulate to obtain wet granules; drying the wet granules, and mixing them with lubricating Agents are mixed and compressed into tablets to obtain Cistanche Chewable Tablets.
[0041] In the present invention, other raw and auxiliary materials other than the lubricant are mixed in parts by mass. As an optional embodiment, the raw and auxiliary materials are mixed evenly with a mixing granulator, the rotating speed is 300-400r / min, preferably 320-360r / min; the mixing time is 5-10min, preferably 8min; the cutting speed is 1500 ~2000r / min, more preferably 1700~1900r / min, cutting time is 3~5min, more preferably 4min. The present invention does not limit the specific mixing method, as long as the raw and auxiliary materials are ...
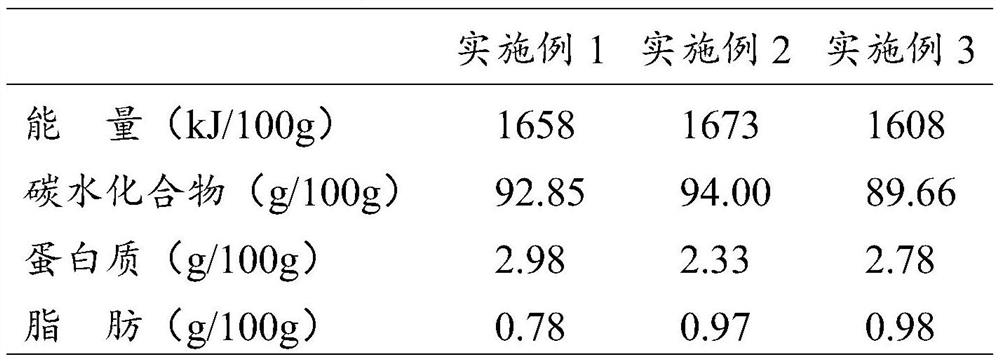
Embodiment 1
[0049] A chewable tablet containing Herba Cistanche, comprising the following raw materials in parts by mass: 30 parts of Herba Cistanche powder, 27 parts of microcrystalline cellulose, 23 parts of mannitol, 14 parts of lactose, 1 part of sodium carboxymethylcellulose, lemon 0.3 parts of steviol glycosides, 0.08 parts of steviol glycosides, 0.2 parts of vitamin C, 0.2 parts of vitamin A, 0.4 parts of vitamin B, and 0.6 parts of magnesium stearate.
[0050] Preparation:
[0051] (1) Preparation of ultrafine powder of Cistanche: fresh slices of Cistanche were dried in a microwave vacuum for 18 minutes at a temperature of 55°C, a vacuum degree of -8Pa, and a power of 20kW to obtain dried slices of Cistanche; When the water content of the material is 3%, the fresh-dried slices of Cistanche are obtained; the fresh-dried slices of Cistanche are put into an ultrafine pulverizer with an ambient humidity of 40%, and pulverized for 8 minutes, and the pulverized particle size is less tha...
Embodiment 2
[0058] A chewable tablet containing Herba Cistanche, comprising the following raw materials in parts by mass: 20 parts of Herba Cistanche Powder, 21 parts of 50% Poria cocos polysaccharide extract, 25 parts of microcrystalline cellulose, 20 parts of mannitol, 12 parts of lactose, carboxylate 0.6 parts of sodium methylcellulose, 0.3 parts of citric acid, 0.08 parts of steviol glycosides, 0.2 parts of vitamin C, 0.2 parts of vitamin A, 0.2 parts of vitamin B, and 0.5 parts of magnesium stearate.
[0059] Preparation:
[0060] (1) The preparation of Cistanche superfine powder is the same as in Example 1.
[0061] (2) 50% Poria cocos polysaccharide extract is pulverized and passed through a 100-mesh sieve for later use.
[0062] (3) The preparation of auxiliary materials, mixing and granulation, drying, tableting, and sterilization packaging methods are the same as in Example 1.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Granularity | aaaaa | aaaaa |
| Granularity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com