IFA neutralizing antibody detection method of PRRSV
An antibody detection and cell technology, applied in the biological field, can solve the problems of inability to evaluate PRRSV-specific antibody response and development
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0027] Embodiment 1PRRSV separation and identification
[0028] Rinse the suspected PRRSV disease tissue (lymph, liver, spleen, lung) with PBS, cut it into pieces, add 1mL PBS to 1g of the tissue sample, freeze and thaw three times, and make the disease material grinding liquid (used as PRRSV virus liquid), Use the RNA extraction kit to extract the RNA of PRRSV from the above disease materials, and then reverse transcribe the RNA into cDNA.
[0029] Use DNAMAN software to design PRRSV target gene primers, and the specific primer sequences are as follows:
[0030] PRRSV-F: 5'-GGCCAGCCAGTCAATCAG-3'; SEQ ID NO.1;
[0031] PRRSV-R: 5'-GGCAAACTAAACTCCACAGTG-3'; SEQ ID NO.2.
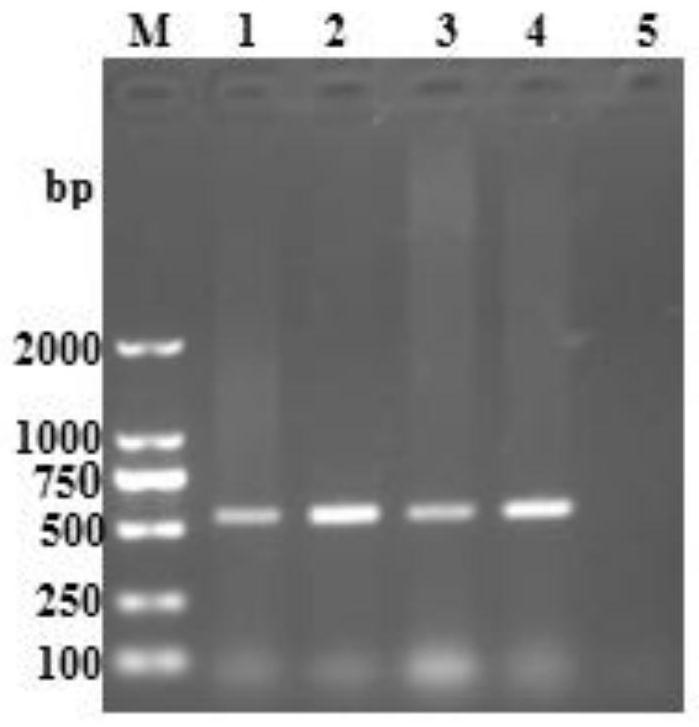
[0032] Using the designed primers, PCR amplification was carried out using the above-mentioned extracted PRRSV lymph, liver, spleen, and lung cDNA as templates. The system was 25 μL, and the reaction conditions were: pre-denaturation at 98°C for 5 minutes; denaturation at 95°C for 30 seconds, annealing at 55...
Embodiment 2
[0033] Example 2 establishes a stable IFA neutralizing antibody detection method
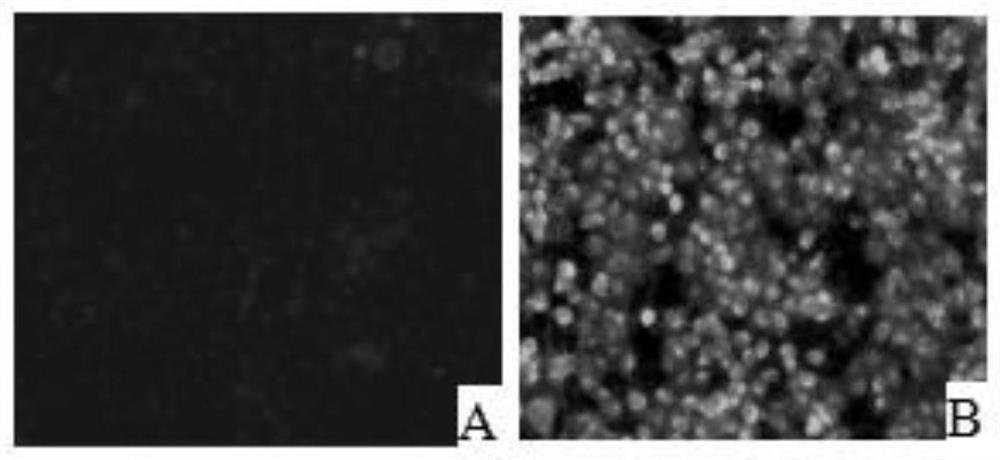
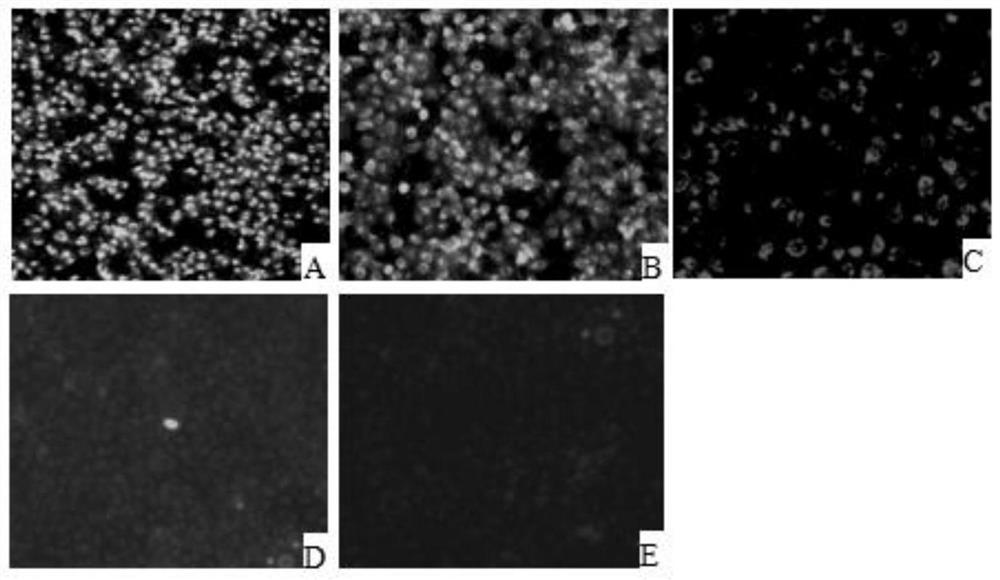
[0034] Marc145 cells were digested and centrifuged to prepare 1.0×10 5 Cells / ml cell suspension, 100 μl per well spread on 96-well plate, placed at 37°C 5% CO 2 Cultivate in the incubator for 12 hours. After the cells are completely adhered to the wall, incubate an equal volume of PRRSV virus solution (50 μl) and clinical serum at 37°C for 1 hour, and then inoculate them on Marc145 cells in a 96-well plate. After culturing for 48 hours, take out after the cells are full. 96-well plate, fixed with pre-cooled absolute ethanol. Add PRRSV standard positive serum, incubate at 37°C for 1h, then add goat anti-pig IgG-FITC, incubate at 37°C for 1h, observe under a fluorescent microscope, negative for specific green fluorescence, otherwise positive. The serum identified as positive was serially diluted, and the above operation was repeated to obtain the antibody titer.
[0035] (1) Plating: Marc145 ce...
Embodiment 3I
[0040] Embodiment 3IFA reaction conditions
[0041] 1) TCID of PRRSV 50 determination
[0042] (1) Spread the Marc145 cell suspension on a 96-well plate, 100 μL per well, so that the cell volume reaches 2-3×10 5 cells / mL, cultivated for 12 hours until the cells were completely attached to the wall;
[0043] (2) In the penicillin bottle or the centrifuge tube, the PRRSV virus liquid is diluted 10 times continuously, starting from 10 -1 -10 -10 ;
[0044] (3) Inoculate the diluted virus onto a 96-well plate in which the cells grow into a single layer, inoculate a vertical row of 8 wells for each dilution, and inoculate 100 μL in each well;
[0045] (4) The remaining two vertical rows are not exposed to poison, and a normal cell control is established (100 μL of maintenance solution per well, and the maintenance solution is a DMEM medium with a fetal bovine serum content of 2%);
[0046] (5) After culturing for 48 hours, the cells were taken out and fixed, and placed at -20...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com