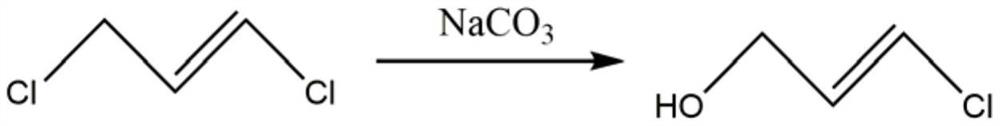Preparation method of trans-3-chloro-2-propenyl hydroxylamine
A technology of propenyl hydroxylamine and dichloropropene, which is applied in the field of preparation of trans-3-chloro-2-propenyl hydroxylamine, can solve the problems of high cost, difficulty in industrialization, and inconvenient operation, and achieve high yield and easy operation. The effect of simplicity and easy purchase of raw materials
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
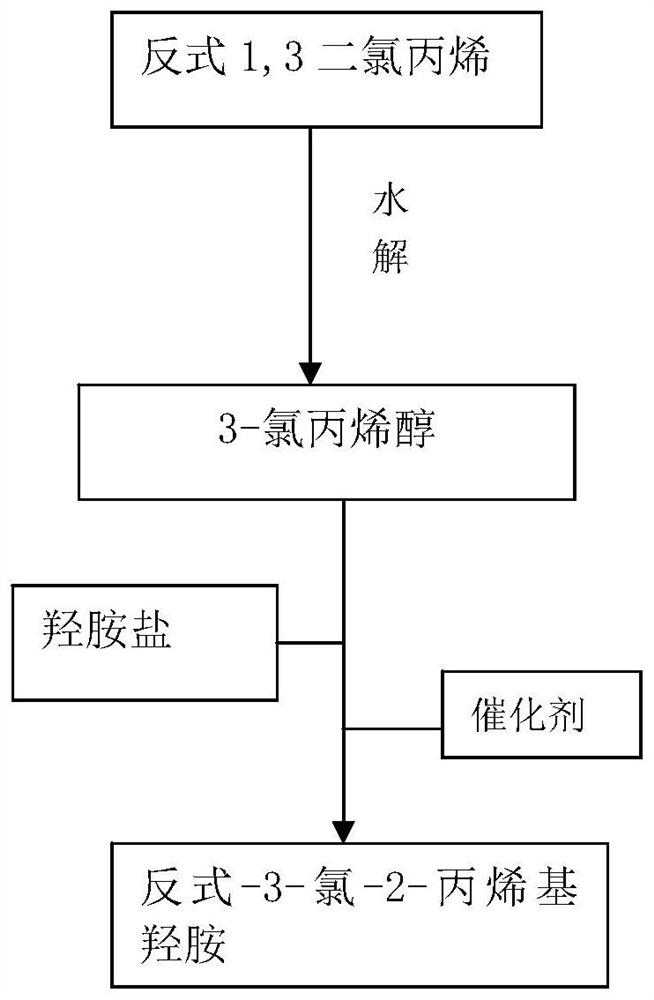
[0024] Such as figure 1 Shown, a kind of preparation method of trans-3-chloro-2-propenyl hydroxylamine of the present invention, described preparation method specifically comprises the following steps:
[0025] S1): using trans-1,3-dichloropropene as raw material, first hydrolyzing trans-1,3-dichloropropene to obtain 3-chloropropenol;
[0026] S2): 3-chloropropenol obtained in S1 and hydroxylamine salt are catalyzed by an acidic catalyst to generate trans-3-chloro-2-propenylhydroxylamine.
[0027] The specific steps of said S1) are:
[0028] S1.1) Now mix trans-1,3-dichloropropene and alkali metal carbonate evenly in proportion;
[0029] S1.2) The homogeneously mixed material of S1.1) is subjected to a hydrolysis reaction at a temperature of 98-100° C., and the reaction time is 8-12 hours;
[0030] S1.3) After the completion of the hydrolysis reaction, let it stand for 25-35 minutes, take the upper layer and extract it with ethyl acetate for 3 to 5 times, then distill under...
Embodiment 1
[0042] Put trans-1,3-dichloropropene and potassium carbonate into the reaction flask, raise the temperature to 80°C, and react for 8 hours to obtain an aqueous solution of 3-chloropropenol, then let it stand for 30 minutes, and extract the upper layer with ethyl acetate for 3-5 After that, distill under reduced pressure at 50°C, distill the fraction at 28°C to obtain 3-chloropropenol; put 3-chloropropenol and hydroxylamine sulfate into the reaction flask, then add sulfonic acid resin for alcohol dehydration reaction, and heat up to 80°C, React for 8 hours, then let stand for 30 minutes, extract and distill to obtain trans-3-chloro-2-propenyl hydroxylamine; the molar ratio of trans-1,3-dichloropropene and potassium carbonate is 1:0.7; the 3- The molar ratio of chloropropenol to hydroxylamine sulfate is 1:1.2.
[0043] The yield of trans-3-chloro-2-propenyl hydroxylamine obtained in this example was 81%, and the purity was 89%.
Embodiment 2
[0045] Put trans-1,3-dichloropropene and sodium carbonate into the reaction flask, raise the temperature to 90°C, and react for 10 hours to obtain an aqueous solution of 3-chloropropenol, then let it stand for 30 minutes, and extract the upper layer with ethyl acetate for 3-5 After that, distill under reduced pressure at 50°C, and distill the fraction at 28°C to obtain 3-chloropropenol; put 3-chloropropenol and hydroxylamine hydrochloride into the reaction flask, then add sulfonic acid resin for alcohol dehydration reaction, and heat up to 90°C. Reacted for 10 hours, then stood still for 30 minutes, and extracted and distilled to obtain trans-3-chloro-2-propenyl hydroxylamine; the molar ratio of the trans-1,3 dichloropropene and potassium carbonate was 1:0.9; the 3-chloro The molar ratio of allyl alcohol to hydroxylamine sulfate is 1:1.5.
[0046] The yield of trans-3-chloro-2-propenyl hydroxylamine obtained in this example was 85%, and the purity was 99.2%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com