LC-MS/MS determination method for residual quantity of rimantadine in egg
An LC-MS, rimantadine technology, applied in the field of determination of veterinary drug residues in eggs, to avoid matrix interference, good repeatability and high sensitivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
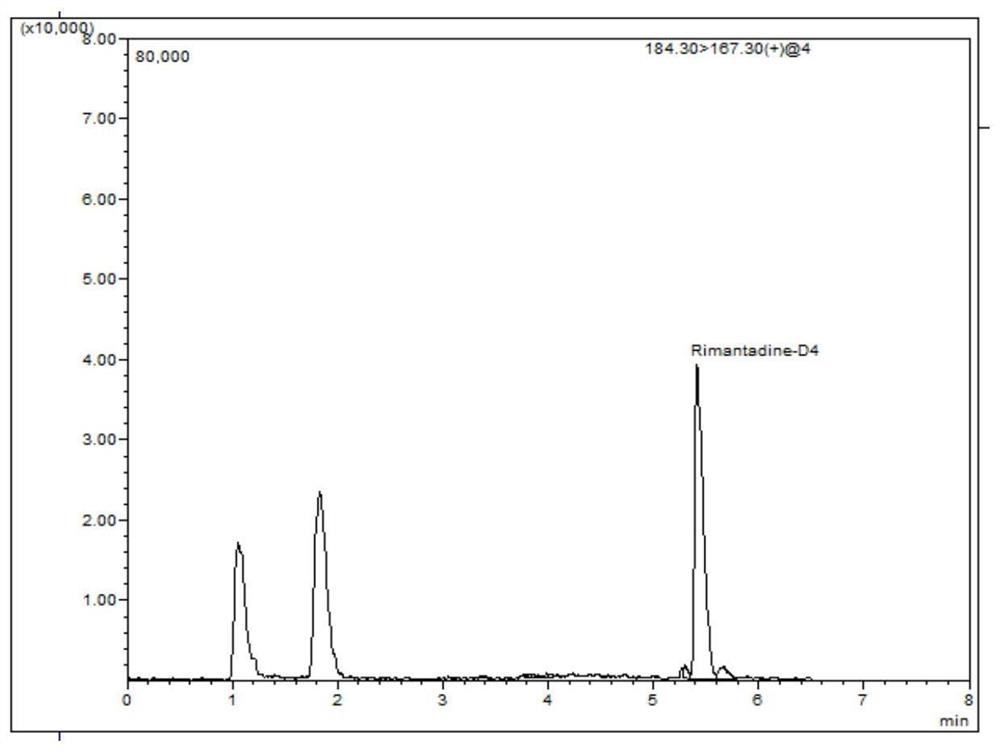
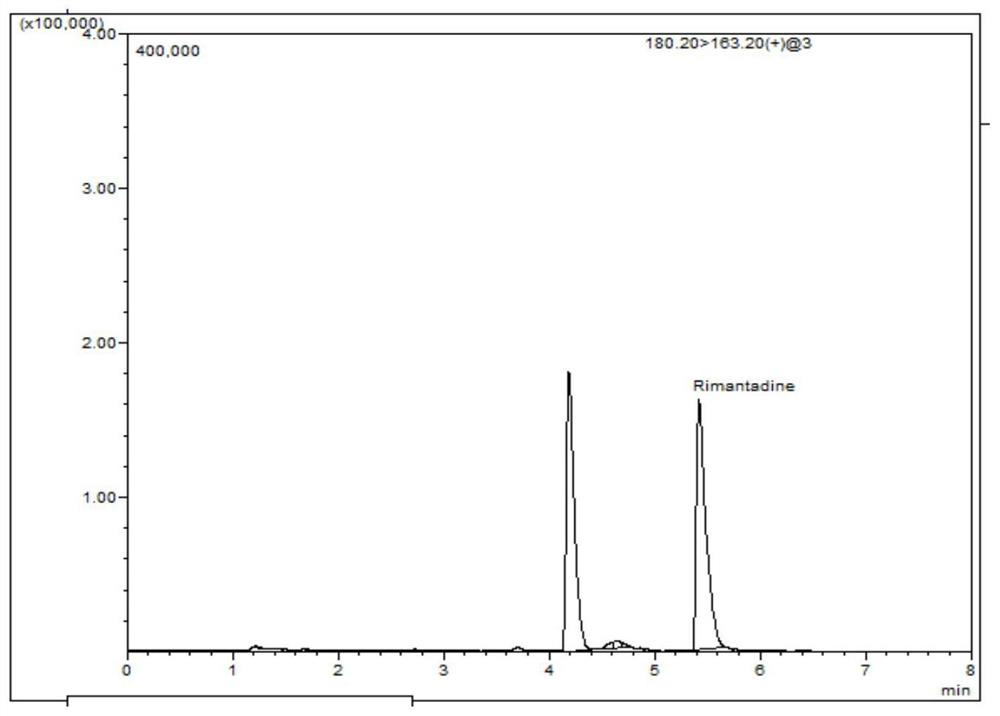
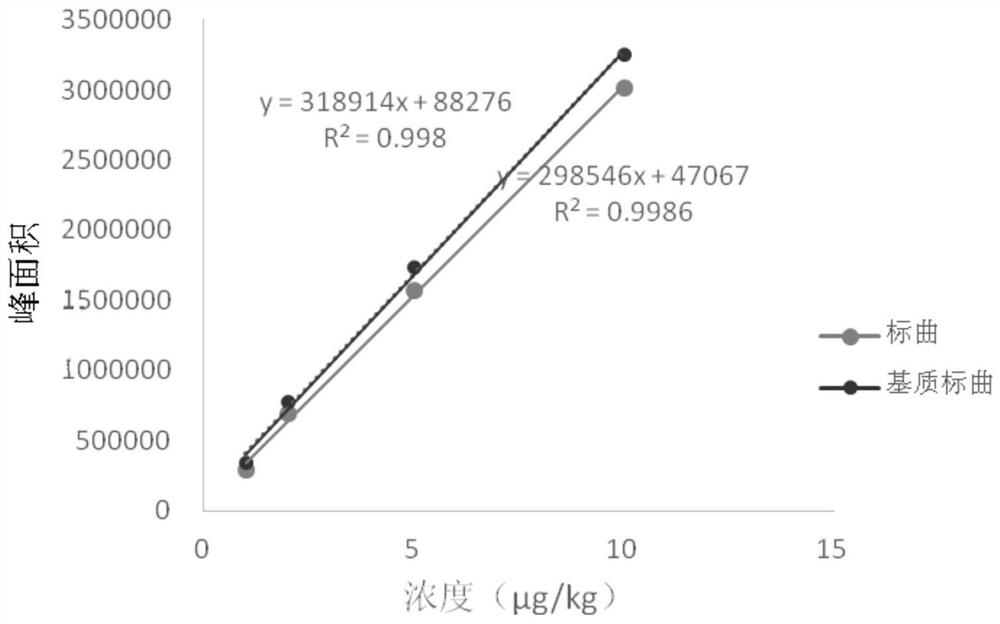
[0054] Example 1: Detection of rimantadine residues in eggs
[0055] (1) extraction
[0056] Take the eggs to be tested, break them, stir the egg yolks and egg whites evenly with a homogenizer, weigh 2±0.02g samples into a 50mL centrifuge tube, add 100μL of 20μg / L internal standard, mix well, let stand for 30min, add 1% Acetic acid acetonitrile solution 10mL, vortex 2min, centrifuge at 10000r / min for 5min, transfer the supernatant to a 50mL centrifuge tube, repeat the extraction once, combine the supernatant twice, add n-hexane 15mL, vortex 1min, 10000r / min Centrifuge for 10 min, discard the n-hexane layer, and set aside.
[0057] (2) purification
[0058] Mixed cation solid-phase extraction column Oasis MCX was activated with 3mL methanol, equilibrated with 3mL water, then the supernatant was put on the column, the flow rate was controlled at 2-3 drops / s, rinsed with 3mL 2% hydrochloric acid aqueous solution, 3mL methanol, and drained 3min, eluted with 5mL 5% ammonia water...
Embodiment 2
[0088] Example 2 Determination method
[0089] The qualitative determination is qualitative by comparing the retention time of the sample chromatogram with the retention time of the standard, and the characteristic ions of the chromatographic peak and the characteristic ions of the chromatographic peak of the corresponding concentration standard. The relative deviation of the retention time between the sample and the standard is not more than 2.5%; the relative abundance of the characteristic ion of the sample is consistent with the relative abundance of the standard solution with equivalent concentration, and the relative abundance deviation does not exceed the provisions in Table 3, then it can be judged that there is corresponding measured object. The deviation of the retention time is within ±5%, and the relative abundance of the detected ions should be consistent with the relative abundance of the calibration standard solution with equivalent concentration. Its allowable...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com