Analogue of Nanocystin A as well as preparation method and application of analogue
A technology of analogues and compounds, applied in the field of analogues of NannocystinA and its preparation, can solve the problems of limited natural product sources and difficulty in obtaining sufficient quantities of compounds, and achieve the effects of simple structure, short synthetic route and high target yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
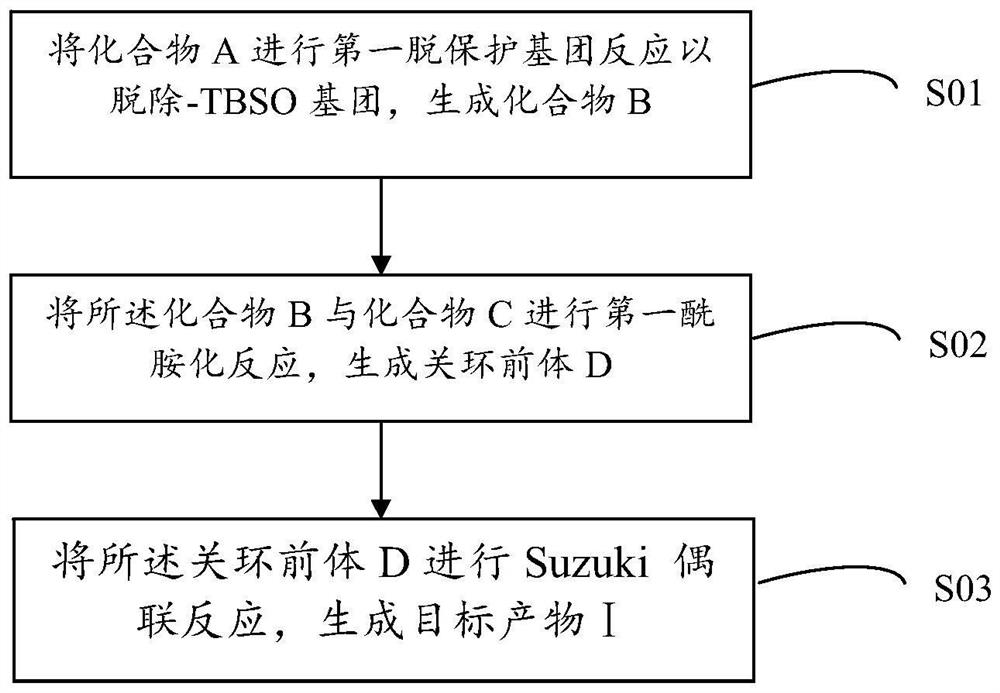
[0048] Therefore, based on the above-mentioned retrosynthetic analysis, the preparation method process of the analogue of Nannocystin A in the above invention embodiment is as follows figure 1 shown, including the following steps:
[0049] S01: Compound A is subjected to the first deprotection group reaction to remove -TBS group and -TMSE group to generate compound B;
[0050] S02: performing a first amidation reaction on compound B and compound C in step S01 to generate ring-closing precursor D;
[0051] S03: performing a Suzuki coupling reaction on the ring-closing precursor D to generate a target product I; wherein the target product I is an analog of Nannocystin A according to any one of claims 1-4;
[0052] According to the preparation method of the analogue of above-mentioned Nannocystin A, its synthetic route is as follows:
[0053]
[0054] Wherein, the first deprotection group reaction in step S01 can follow the method of removing -TBS group (tert-butyldimethylsi...
Embodiment 1
[0084] The embodiment of the present invention provides an analogue of Nannocystin A and a preparation method thereof. Wherein, the molecular structural formula of the analogue of Nannocystin A is shown in the following Ia.
[0085] The analog Ia preparation method of the Nannocystin A comprises the following steps:
[0086] S1. Synthesis of compound 9a:
[0087]
[0088] At 0°C, compound 6 (268 mg, 0.96 mmol) was dissolved in DCM (6 mL), and PyAOP (834 mg, 1.6 mmol) and DIPEA (0.7 mL, 4.0 mmol) were gradually added to the reaction system. The fully deprotected acid (0.8 mmol) was then dissolved in DCM (2 mL) and added to the reaction. The reaction was warmed to room temperature and stirring was continued for 12 hours. saturated NH 4 Cl solution (20 mL) quenched the reaction. Repeated extraction with ethyl acetate (3 x 30 mL). The combined organic phases were sequentially washed with saturated NaHCO 3 solution (30mL) and saturated brine (30mL), washed with anhydrous ...
Embodiment 2
[0101] The embodiment of the present invention provides an analogue of Nannocystin A and a preparation method thereof. Wherein, the molecular structural formula of the analogue of Nannocystin A is shown in Ib below.
[0102] The analog Ib preparation method of the Nannocystin A comprises the following steps:
[0103] S1. Synthesis of compound 5b':
[0104]
[0105] Paraformaldehyde (2.6g, 29.3mmol) and p-toluenesulfonic acid (77.5mg, 0.45mmol) were sequentially added to a toluene solution (45mL) of compound 13 (2g, 4.5mmol), and heated to reflux for 4 hours. After the reaction was complete, it was cooled to room temperature and extracted repeatedly with ethyl acetate (3 x 30 mL). Combine the organic phases and wash with NaHCO 3 Saturated solution (30mL) washed, anhydrous Na 2 SO 4 dry. After spin-drying, put it directly into the next step of reaction;
[0106] The obtained five-membered ring compound (1.5g, 3.3mmol) was dissolved in DCM (10mL), TFA (5.4mL, 72.6mmol) ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com