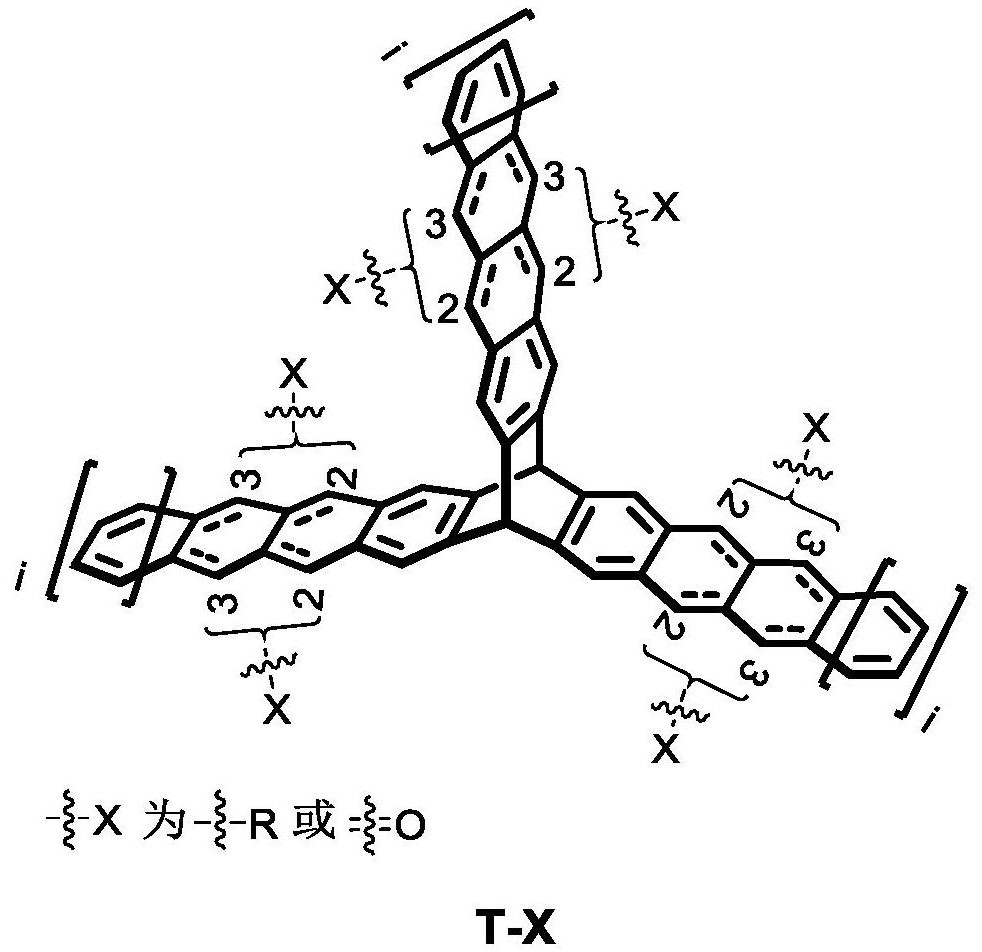
Linear acene compound based on triptycene and synthesis and application thereof
A compound, the technology of triptycene, applied in the field of linear acene compounds and their synthesis and application, can solve the lack of trimer molecular design, performance research and application, and research on the synthesis and application of acene trimer compounds Issues not reported
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
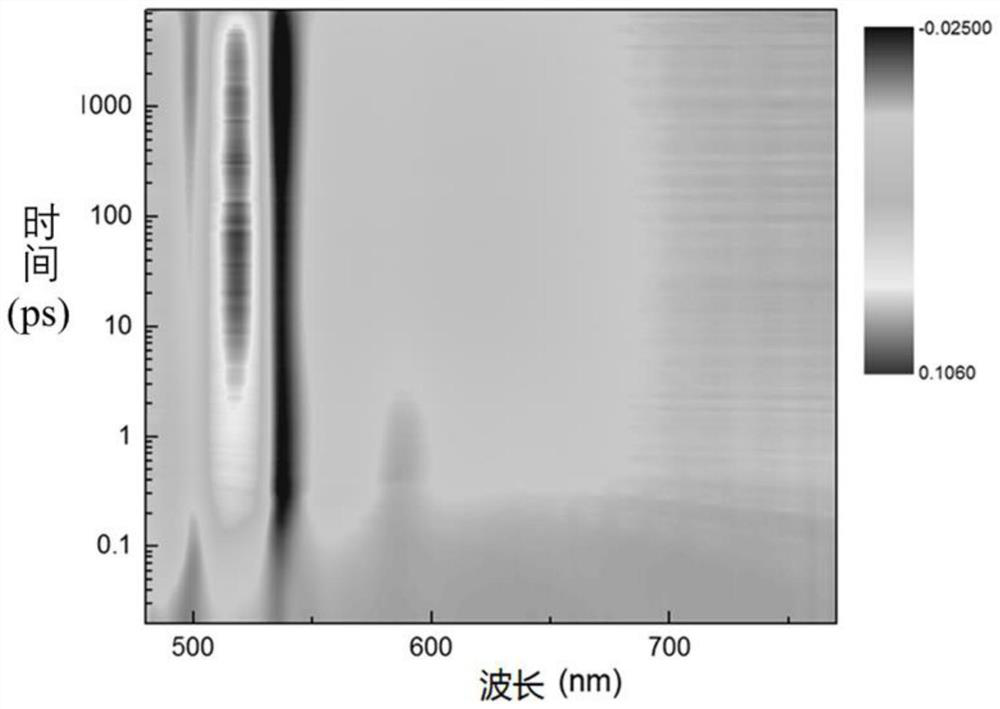
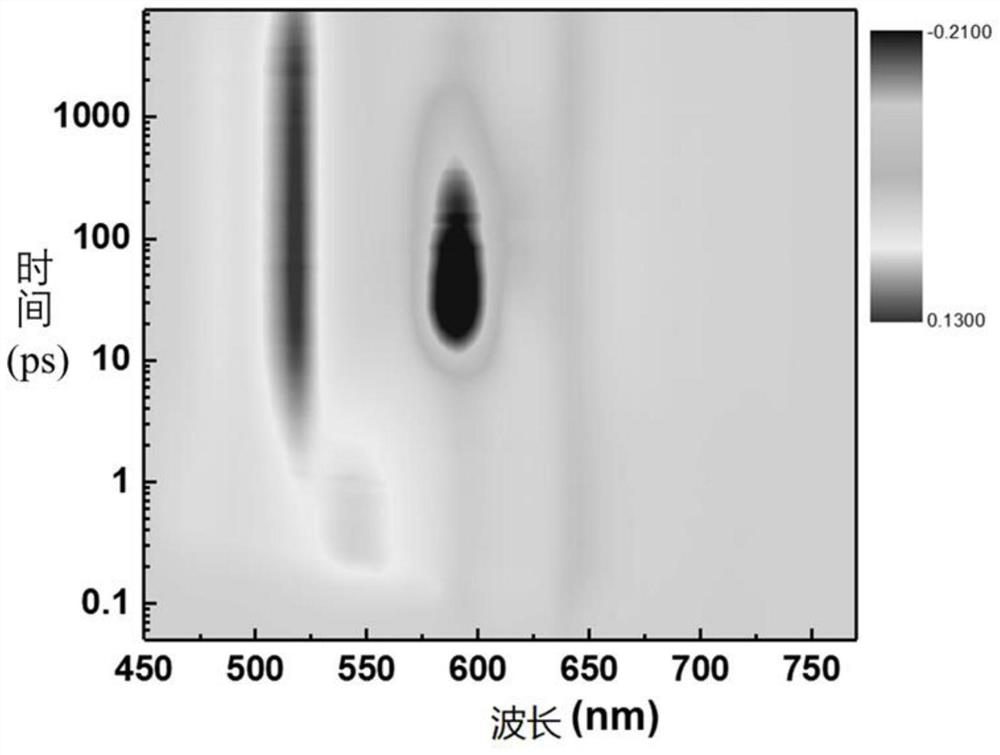
Image
Examples
Embodiment 1
[0077] This embodiment synthesizes compound 2-1 and 3-1-1
[0078] Synthesis of compound 1-1
[0079] Under the condition of nitrogen protection, triptycene (1.0g, 3.9mmol), phthalic anhydride (3.7g, 24.9mmol) and 50mL tetrachloroethylene were added to a 100mL three-necked flask, and after stirring evenly, the trichloro Aluminum chloride (6.5 g, 48.7 mmol) was added slowly. Heated to reflux at 115°C for 16h. After the reaction was complete, it was cooled to room temperature, poured into 5% hydrochloric acid in ice water, and stirred for 30 min. The solid was filtered, washed with water, and dried in vacuo. The obtained crude product was dissolved in 100 mL of acetonitrile, filtered, and the filtrate was spin-dried to obtain triptylenonic acid derivative 1-1 (2.3 g), with a yield of 84%. Triptylenonic acid derivative 1-1 is a mixture of two stereoisomers, which is directly used in the next reaction.
[0080]
[0081] Synthesis of Compound 2-1
[0082] Compound 1-1 (1g,...
Embodiment 2
[0085] This embodiment synthesizes compound 3-1-1
[0086] Synthesis of compound 3-1-1
[0087] Under the condition of nitrogen protection, triisopropylsilylacetylene (4.2mL, 18.7mmol) was dissolved in 10mL ether, cooled to 0°C, and then 3.3mL n-butyllithium (2.5M) was slowly dissolved in 30min Drop into the mixture and stir at 0°C for 1h. Then, a mixed suspension of compound 2-1 (0.5 g, 0.78 mmol) in tetrahydrofuran (10 mL) was added in one portion, and then the mixture was raised to room temperature and stirred for 12 h. After the reaction was complete, 10 mL of a saturated stannous chloride solution of 10% hydrochloric acid was added dropwise to the reaction solution, and stirring was continued for 1 h. The liquid was separated, and the upper organic phase was dried with anhydrous sodium sulfate, and the solvent was removed by rotary evaporation. Using pure petroleum ether as the developer for column chromatography separation, the light yellow product 3-1-1 (319 mg) was ...
Embodiment 3
[0090] This embodiment synthesizes compound 3-1-3
[0091] Synthesis of compound 3-1-3
[0092] Under nitrogen protection, 2,6-diisopropylbromobenzene (4.5g, 18.7mmol) was dissolved in 10mL of ether, cooled to 0°C, and then 3.3mL of n-butyllithium (2.5 M) slowly drop into the mixture, and stir at 0°C for 1 h. Then, a mixed suspension of compound 2-1 (0.5 g, 0.78 mmol) in tetrahydrofuran (10 mL) was added in one portion, and then the mixture was raised to room temperature and stirred for 12 h. After the reaction was complete, 10 mL of a saturated stannous chloride solution of 10% hydrochloric acid was added dropwise to the reaction liquid, and stirring was continued for 1 h. The liquid was separated, and the upper organic phase was dried with anhydrous sodium sulfate, and the solvent was removed by rotary evaporation. Using pure petroleum ether as the developer for column chromatography separation, the light yellow product 3-1-3 was obtained.
[0093]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com