Synthesis method of 3-methyl-2-butene-1-aldehyde diisopentenyl acetal
A technology of aldehyde diprenyl acetal and synthesis method, which is applied in chemical instruments and methods, preparation of organic compounds, organic compounds/hydrides/coordination complex catalysts, etc., can solve the problem of increasing production costs and usage It is difficult to control and requires high production equipment, so as to achieve the effect of extending service life, significant environmental protection benefits, and avoiding equipment corrosion.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
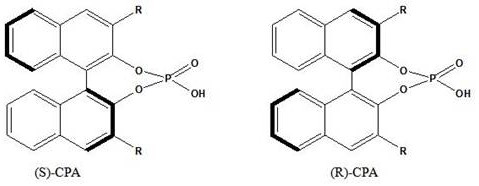
[0048] Place a certain amount of HZIF-8 material in a vacuum oven at 80°C and dry for 10 hours to remove all the moisture in the carrier until the carrier is completely dry. Accurately weigh 10 g of the dried carrier, add it into 50 g of toluene, disperse evenly, heat to 80°C, keep warm for 12 hours, filter, wash, and vacuum dry at 70°C until constant weight. At 70°C, evenly disperse the pretreated carrier into 100g PEG400, stir at a constant speed, and after 2 hours of heat preservation, add 0.9g (R)-CPA (the two groups R are both 4-F 3 CC 6 h 4 ), raise the temperature to 80°C, keep warm for 4 hours, cool to room temperature, filter, and dry to constant weight at 80°C to obtain the catalyst, marked as 9.0wt.% 4-F 3 CC 6 h 4 -(R)-CPA / HZIF-8 (the loading here is the loading relative to the dry carrier mass).
[0049] Using the same method as above, change the configuration of chiral phosphoric acid and the type of R group, the type and ratio of carrier and surface modifie...
Embodiment 27
[0061] Example 27 Catalyst application
[0062] For catalyst 9.0 wt.% 4-F 3 CC 6 h 4 -(R)-CPA / HZIF-8 Carried out the experiment of catalyst application to investigate the stability of the catalyst. 9.0 wt.% 4-F in Example 14 3 CC 6 h 4 -The catalyst of (R)-CPA / HZIF-8 is used to catalyze the synthesis of 3-methyl-2-butene-1-aldehyde diisopentenyl acetal according to the method of the above-mentioned catalyst performance test, after 3000 hours of continuous use , when the catalyst activity is lower than 70%, regenerate and activate the catalyst, and then continue to apply it. The specific operation is the same as that in the catalyst performance test. The specific experimental data are shown in Table 3.
[0063] The regeneration and activation method of the catalyst is as follows: uniformly disperse the catalyst in a mixed solvent of toluene and ethanol, stir at 80°C for 5 hours, filter, and vacuum-dry at 80°C until constant weight.
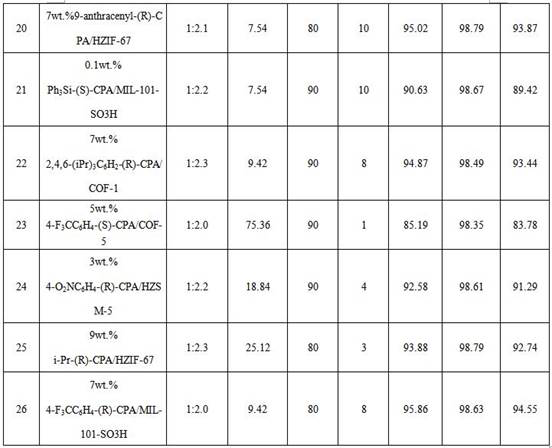
[0064] Table 3 Application data
[...
Embodiment 28
[0068] Example 28 using 9.0 wt.% 4-F 3 CC 6 h 4 -(R)-CPA / HZIF-8 as a catalyst for the reaction
[0069] In a glass beaker, mix prenaldehyde and prenol at a molar ratio of 1:2, add 9.0 wt.% 4-F 3 CC 6 h 4 -(R)-CPA / HZIF-8 3.0wt.% (accounting for the total amount of prenol and prenal) as a catalyst, after mixing evenly, set aside.
[0070] Add the mixed liquid into a 500ml stainless steel reaction kettle, replace with nitrogen 3 times, start stirring and heating, the stirring speed is 400 rpm, the temperature is set at 80°C, and the reaction is kept at normal pressure for 2 hours. The reaction solution was detected by gas chromatography, and the conversion and selectivity were calculated. The results are: the conversion rate of prenaldehyde is 98.75%, the selectivity of 3-methyl-2-butene-1-aldehyde diprenyl acetal is 99.26%, and the selectivity of 3-methyl-2-butene- The crude product yield of 1-aldehyde di-isopentenyl acetal is 98.02%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com