Preparation method of balosavir intermediate
An intermediate and central control technology, applied in the field of medicine, can solve the problems of high cost, poor product purity and chromaticity, long steps, etc., and achieve the effect of facilitating industrial production and good product quality
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
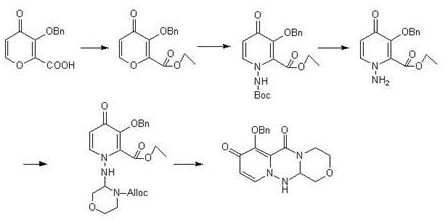
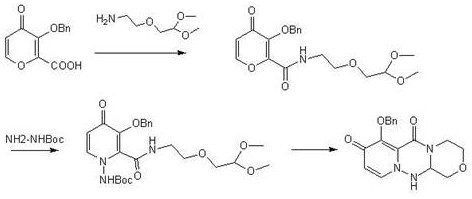
[0029] A kind of preparation method of baloxavir intermediate specifically comprises the following steps:
[0030] (1) Add 1L tetrahydrofuran to the reaction flask, add 1.68kg dicyclohexylcarbodiimide, 1kg 3-(benzyloxy)-4-oxo-4H-pyran-2-carboxylic acid and 1.5kg 2, -(2-Aminoethoxy)-1,1-dimethoxyethane, start stirring, raise the temperature to 60°C for reaction, and track with central control. After completion of the reaction, cool down to room temperature, add 3L of water, then extract with 5L of ethyl acetate, and concentrate the organic phase to obtain 1.33kg of Intermediate I, with a yield of 87%.
[0031] (2) Add 4L of N,N-dimethylacetamide into the reaction flask, then add 1.33kg of intermediate I and 2.3kg of pyridinium p-toluenesulfonate, start stirring, raise the temperature to 60°C, and drop tert-butoxycarbonylhydrazine 0.6 kg and 2L N,N-dimethylacetamide mixed solution, temperature control 60 ℃ reaction, central control tracking. After the reaction was completed, l...
Embodiment 2
[0034] A kind of preparation method of baloxavir intermediate specifically comprises the following steps:
[0035] (1) Add 1L 1,4-dioxane to the reaction flask, add 0.63kg 1-ethyl-3(3-dimethylpropylamine) carbodiimide, 1kg 3-(benzyloxy)-4- Oxo-4H-pyran-2-carboxylic acid and 0.6kg 2,-(2-aminoethoxy)-1,1-dimethoxyethane, started stirring, raised the temperature to 80°C to react, and followed the central control. After the reaction was completed, cool down to room temperature, add 3L of water, then extract with 5L of ethyl acetate, and concentrate the organic phase to obtain 1.30kg of Intermediate I.
[0036] (2) Add 4L of N,N-dimethylformamide into the reaction flask, then add 1.30kg of intermediate I and 2.3kg of pyridinium p-toluenesulfonate, start stirring, raise the temperature to 80°C, and drop tert-butoxycarbonylhydrazine 0.6 kg and 2L N,N-dimethylformamide mixed solution, temperature control 80 ℃ reaction, central control tracking. After the reaction was completed, lowe...
Embodiment 3
[0039] A kind of preparation method of baloxavir intermediate specifically comprises the following steps:
[0040] (1) Add 1L N,N-dimethylformamide, 1.02kg diisopropylcarbodiimide, 1kg 3-(benzyloxy)-4-oxo-4H-pyran- 2-Carboxylic acid and 1.5kg 2,-(2-aminoethoxy)-1,1-dimethoxyethane, start stirring, raise the temperature to 50°C and react with central control. After completion of the reaction, cool down to room temperature, add 3L of water, then extract with 5L of ethyl acetate, and concentrate the organic phase to obtain 1.33kg of Intermediate I.
[0041] (2) Add 4L of N,N-dimethylacetamide into the reaction flask, then add 1.33kg of intermediate I and 2.3kg of pyridinium p-toluenesulfonate, start stirring, raise the temperature to 80°C, and drop tert-butoxycarbonylhydrazine 0.6 kg and 2L N,N-dimethylacetamide mixed solution, temperature control 80 ℃ reaction, central control tracking. After the reaction was completed, lower the temperature to 0°C, add 2L of ethanol and 8L of...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com