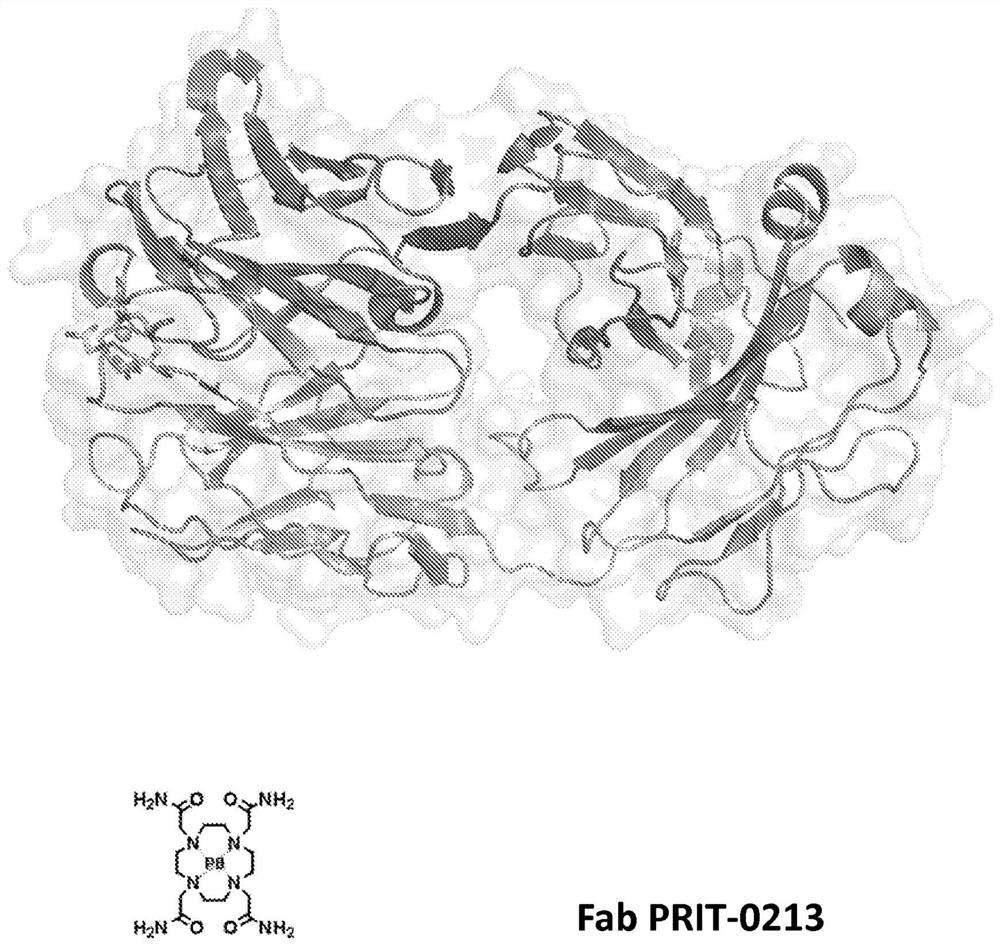
Antibodies for chelated radionuclides
A bispecific antibody, antibody technology, applied in the field of scavengers and compositions, bispecific antibodies, can solve the problems of slow complex formation rate, unstable binding of radionuclides, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0866] Example 1: Description of Immunization
[0867] Immunization of Rabbits
[0868] The 2 enantiomers Pb-DOTAM-Alkyl-PEG 4 - 1:1 mixture of KLH fractions (MS2-DOTAM KLH fraction 1 and MS2-DOTAMKLH fraction 2) for immunization of New Zealand white rabbits or transgenic rabbits containing human immunoglobulin loci, as in WO 2000 / 46251, WO 2002 / 12437, WO 2005 / 007696, WO 2006 / 047367, US 2007 / 0033661, and WO 2008 / 027986. Each rabbit was immunized with 500ug of the immunogen mixture emulsified in complete Freund's adjuvant by intradermal application on day 0 and 500ug each day by alternating intramuscular and subcutaneous application on days 7, 14, 28, 56 . Thereafter, rabbits received monthly subcutaneous immunizations of 500ug, and aliquots of blood were collected 7 days after immunization for serum titer determination. Larger blood samples (estimated 10% of total blood volume) were collected during month 3 of immunization and during month 9 (5-7 days after immunization...
Embodiment 2
[0871] Example 2: Cloning B cells from rabbits
[0872] Isolation of Rabbit Peripheral Blood Mononuclear Cells (PBMC)
[0873] Blood samples were obtained from immunized rabbits. Whole blood containing EDTA was diluted two-fold with 1x PBS (PAA, Pasching, Austria), followed by density centrifugation using mammalian lymphocytes (Cedarlane Laboratories, Burlington, Ontario, Canada) according to the manufacturer's instructions. PBMCs were washed twice with 1x PBS.
[0874] EL-4 B5 medium
[0875] Supplemented with 10% FCS (Hyclone, Logan, UT, USA), 2 mM Glutamin, 1% penicillin / streptomycin solution (PAA, Pasching, Austria), 2 mM sodium pyruvate, 10 mM HEPES (PAN Biotech, Aidenbach, Germany) and RPMI 1640 (Pan Biotech, Aidenbach, Germany) in 0.05 mM b-mercaptoethanol (Gibco, Paisley, Scotland).
[0876] coating of the board
[0877] Sterile cell culture 6-well plates were coated with 2 μg / ml KLH in carbonate buffer (0.1 M sodium bicarbonate, 34 mM sodium bicarbonate, pH...
Embodiment 3
[0887] Example 3: Expression of Rabbit Antibody
[0888] PCR amplification of V domains
[0889] Total RNA was prepared from B cell lysates (resuspended in RLT buffer, Qiagen, cat. no. 79216) using the NucleoSpin 8 / 96 RNA kit (Macherey & Nagel; 740709.4, 740698) according to the manufacturer's protocol. RNA was eluted with 60 μl of RNase-free water. cDNA was generated by reverse transcriptase reaction using 6 μl RNA using Superscript III first strand synthesis SuperMix (Invitrogen 18080-400) and oligo dT primers according to the manufacturer's instructions. Implement all steps on the Hamilton ML Star system. Primers rbHC.up and rbHC.do for heavy chain and rbLC.up and rbLC.do for light chain (Table 3) were amplified using 4 μl cDNA in a final volume of 50 μl with AccuPrime Super Mix (Invitrogen 12344-040) Immunoglobulin heavy and light chain variable regions (VH and VL). All forward primers were specific for the signal peptide (VH and VL, respectively), while the reverse ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
| molecular weight | aaaaa | aaaaa |
| molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com