Non-aqueous electrolyte solution, preparation method thereof and lithium ion battery
A non-aqueous electrolyte and lithium-ion battery technology, which is applied in the field of batteries, can solve the problems that the low-temperature discharge performance of the battery needs to be further improved, and achieve the effects of improving the normal temperature cycle of the battery, avoiding further contact, good rate performance and low-temperature discharge performance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
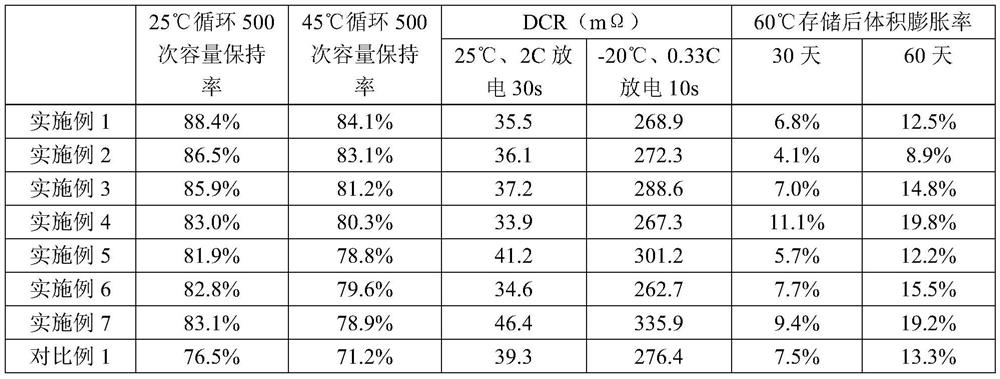
Embodiment 1
[0047] This embodiment provides a non-aqueous electrolytic solution, which is composed of cyclic carbonates, chain carbonates, lithium salts and additives.
[0048] The additive is composed of a sulfonic anhydride additive and a non-sulfonic anhydride additive, the sulfonic anhydride additive is methylsulfonic anhydride, and the non-sulfonic anhydride additive is 1,4-butane sultone. The cyclic carbonate is ethylene carbonate, the chain carbonate is dimethyl carbonate, and the lithium salt is LiPF 6 .
[0049] The mass ratio of the cyclic carbonate and the chain carbonate is 1:2.5; the molar concentration of the lithium salt in the mixed solvent formed by the cyclic carbonate and the chain carbonate is 1.3mol / L; the sulfur The quality of anhydride additives is 0.5% of the total mass of lithium salts, cyclic carbonates and chain carbonates; the quality of non-sulfonic anhydride additives is the lithium salt, cyclic carbonates and chain carbonates 3.0% of the total mass.
[00...
Embodiment 2
[0053] This embodiment provides a non-aqueous electrolytic solution, which is composed of cyclic carbonates, chain carbonates, lithium salts and additives.
[0054] The additive is composed of sulfonic anhydride additives and non-sulfonic anhydride additives, the sulfonic anhydride additive is 2-thiophenesulfonic anhydride, and the non-sulfonic anhydride additives are 1,3-propane sultone and 1,4 - Butane sultone (the mass ratio of 1,3-propane sultone and 1,4-butane sultone is 1:2). The cyclic carbonate is γ-butyrolactone, the chain carbonate is ethyl methyl carbonate, and the lithium salt is LiPF 6 .
[0055] The mass ratio of the cyclic carbonate and the chain carbonate is 1:2; the molar concentration of the lithium salt in the mixed solvent formed by the cyclic carbonate and the chain carbonate is 1.0mol / L; the sulfur The quality of acid anhydride additives is 0.1% of the total mass of said lithium salt, cyclic carbonate and chain carbonate; the quality of said non-sulfoni...
Embodiment 3
[0057] This embodiment provides a non-aqueous electrolytic solution, which is composed of cyclic carbonates, chain carbonates, lithium salts and additives.
[0058] Described additive is made up of sulfonic anhydride additive and non-sulfonic anhydride additive, and described sulfonic anhydride additive is benzenesulfonic anhydride, and described non-sulfonic anhydride additive is adiponitrile and lithium difluorophosphate (adiponitrile and difluoro The mass ratio of lithium phosphate is 1:1). The cyclic carbonate is propylene carbonate, the chain carbonate is diethyl carbonate, and the lithium salt is LiPF 6 .
[0059] The mass ratio of the cyclic carbonate and the chain carbonate is 1:3; the molar concentration of the lithium salt in the mixed solvent formed by the cyclic carbonate and the chain carbonate is 1.5mol / L; the sulfur The quality of acid anhydride additives is 1.0% of the total mass of said lithium salt, cyclic carbonate and chain carbonate; the quality of said ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com