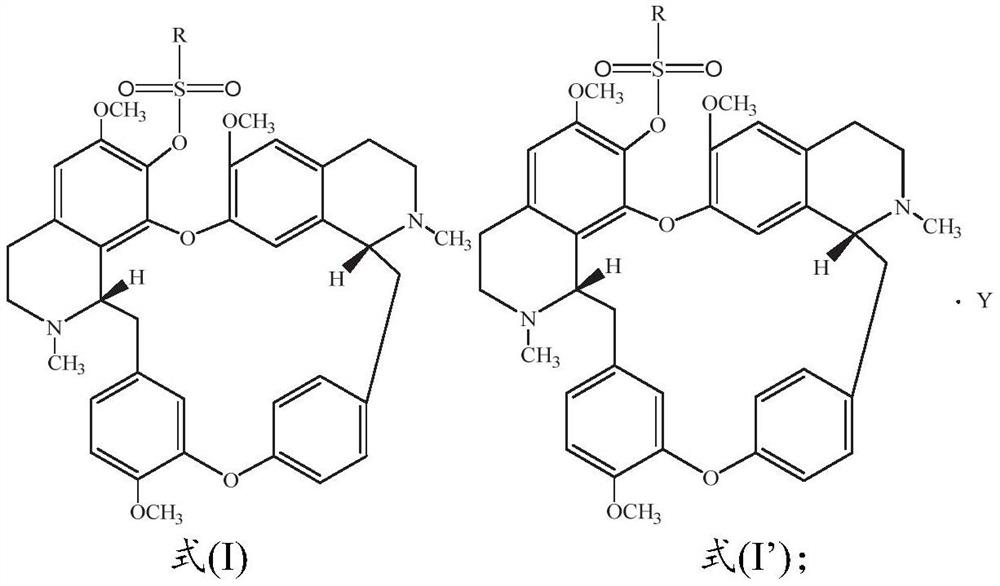
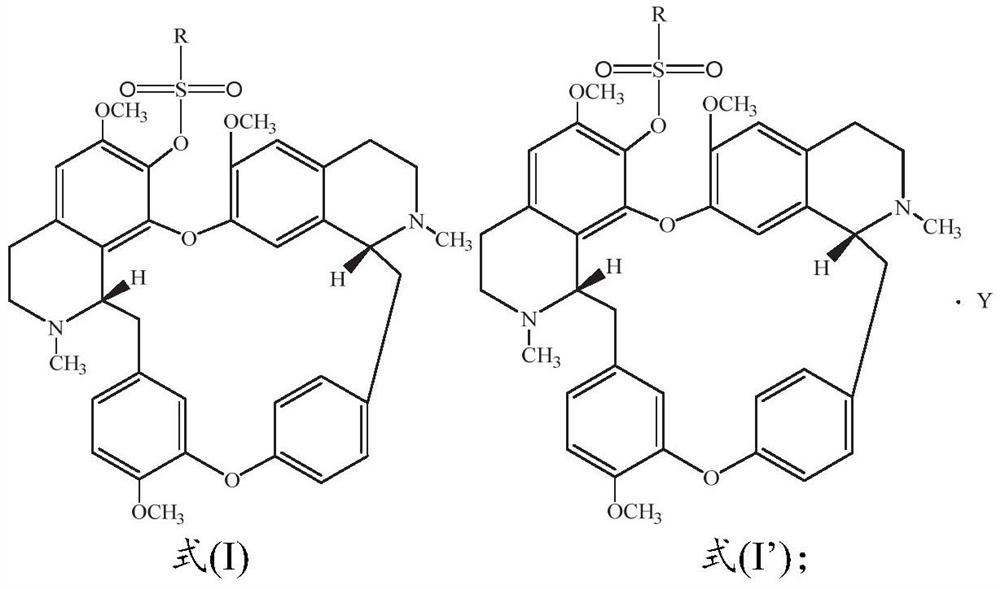
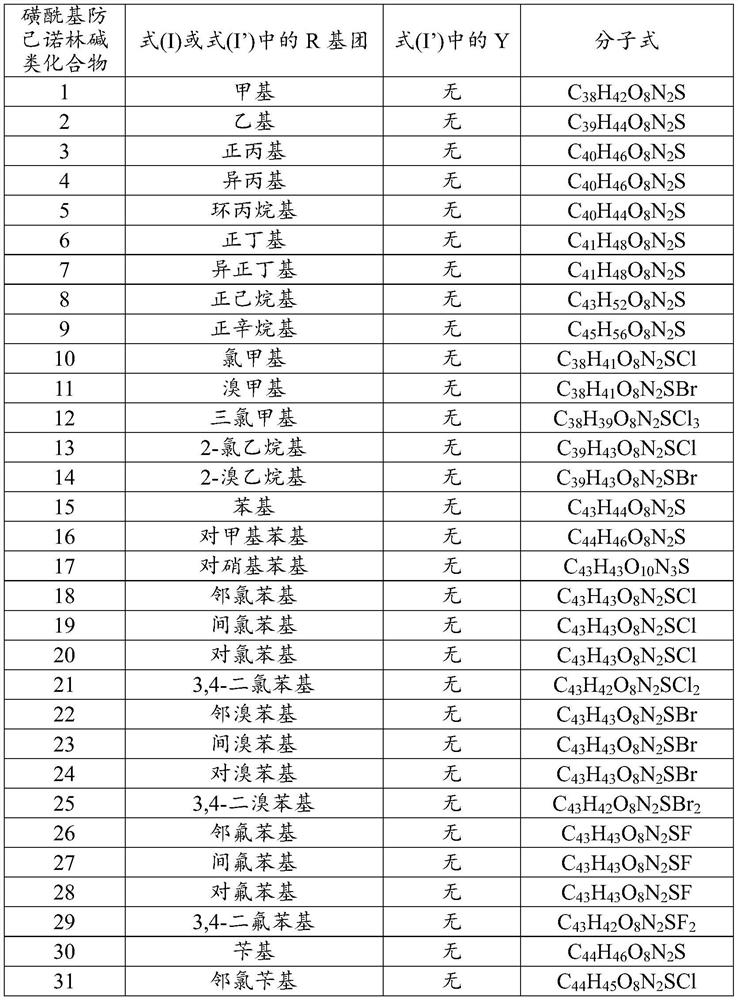
Sulfonyl fangchinoline compound as well as preparation method and application thereof
A technology of fangchinoline base and compound, which is applied in the field of medicine and can solve the problems of small application range
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0055] Dissolve 100.0mg fangchinoline base (about 0.18mmol) in 3mL of dichloromethane, add it into a two-necked flask filled with argon, and place it in a cold well with temperature controlled to 0°C, add the dissolved 2mL under magnetic stirring 66.5 mg (about 0.72 mmol) of triethylamine in dichloromethane was mixed for 1 h, and then 31.0 mg (about 0.27 mmol) of methanesulfonyl chloride was added to react, and the reaction was tracked by TLC. After the reaction was finished, the reaction solution was concentrated and purified through a neutral alumina column. The eluent was dichloromethane:methanol (120:1, 80:1 were used for elution, respectively), and the white powder product 117.5 was separated and purified. mg (about 0.17 mmol), melting point: 208.1-209.9°C, yield 94.4%. Product analysis and characterization: 1 H NMR (400MHz, CDCl 3 )δ7.40(dd, J=8.2,1.9Hz,1H),7.15(dd,J=8.2,2.5Hz,1H),6.85(d,J=7.7Hz,2H),6.81(dd,J=8.3 ,2.5Hz,1H),6.56(s,1H),6.46(s,1H),6.40(s,1H),6.33(dd,J=8...
Embodiment 2
[0057] Dissolve 100.0 mg of fangchinoline base (about 0.18 mmol) in 3 mL of chloroform, add it into a two-necked flask filled with argon, and place it in a cold well with temperature controlled to -20 ° C. Under magnetic stirring, add the dissolved Add 5.0 mg (about 0.20 mmol) of NaH in 2 mL of chloroform, mix for 1 h, add 23.2 mg (about 0.18 mmol) of ethylsulfonyl chloride to react, and track the reaction by TLC. After the reaction was finished, the reaction solution was concentrated and purified through a neutral alumina column. The eluent was dichloromethane:methanol (eluted at 120:1 and 80:1 respectively), separated and purified to obtain a white powdery product 113.6 mg (about 0.162 mmol), melting point: 205.5-207.2°C, yield 90.0%. Product analysis and characterization: 1 H NMR (400MHz, CDCl 3 )δ7.36(dd, J=8.2,1.9Hz,1H),7.14(dd,J=8.2,2.4Hz,1H),6.82(dt,J=8.3,5.3Hz,3H),6.51(d,J =16.7Hz,2H),6.42–6.30(m,2H),6.04(s,1H),3.92(s,3H),3.88–3.81(m,1H),3.81–3.73(m,3H),3.69( s,1H)...
Embodiment 3
[0059] Dissolve 100.0mg fangchinoline base (about 0.18mmol) in 10mL of dichloromethane, add it into a two-necked flask filled with argon, and place it in a cold well with temperature controlled to 0°C. 62.0 mg (about 0.72 mmol) of piperidine in dichloromethane was mixed for 1 hour, then 25.6 mg (about 0.20 mmol) of n-propylsulfonyl chloride was added to react, and the reaction was tracked by TLC. After the reaction, the reaction solution was concentrated to 1 / 3 of the volume of the original solution, crystallized in a cold well at 0°C for more than 4 hours, filtered quickly, and dried in vacuum below 50°C to obtain 115.9 mg (about 0.162 mmol) of a white powder product. Melting point: 204.9-206.5°C, yield 90.0%. Product analysis and characterization: 1 H NMR (400MHz, CDCl 3 )δ7.29(dd, J=8.2,1.9Hz,1H),7.06(dd,J=8.1,2.4Hz,1H),6.74(dt,J=8.3,5.4Hz,3H),6.43(d,J =14.0Hz,2H),6.36–6.19(m,2H),5.96(s,1H),3.84(s,3H),3.76(dd,J=11.1,5.3Hz,0H),3.73–3.65(m, 3H), 3.62(d, J=9.7Hz, 0H), 3.40...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com