Dexamethasone sodium phosphate injection and preparation method thereof
A technology of dexamethasone sodium phosphate and injection, which is applied in the direction of medical formulas, medical preparations with non-active ingredients, medical preparations containing active ingredients, etc., and can solve problems such as blindness, corneal damage, and allergic reactions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0023] The invention provides a dexamethasone sodium phosphate injection and a preparation method thereof, which uses sodium citrate dihydrate and citric acid as auxiliary materials to avoid the safety problems caused by adding organic solvents and antioxidants. The amount of sodium citrate dihydrate and the amount of citric acid are used to ensure the osmotic pressure and pH value of the preparation. The preparation process is achieved by adopting a lower liquid preparation temperature, nitrogen filling, and filtration sterilization process through a filter element with a pore diameter of 0.2 μm. The stability of the preparation is ensured, and the problem of drug stability is solved.
[0024] Further, the preferred formulation of dexamethasone sodium phosphate injection, the injection is calculated by weight percentage, including: dexamethasone sodium phosphate 1.093%, sodium citrate dihydrate 2.59%, citric acid 0.007%, and the balance is injection use water.
[0025] Furth...
Embodiment 1
[0041] Based on the preparation of 1 L of dexamethasone sodium phosphate injection, the dexamethasone sodium phosphate injection includes the following components in mass percentage:
[0042]
[0043] Described preparation process comprises the steps:
[0044] S1, Weighing: Weigh dexamethasone sodium phosphate, sodium citrate dihydrate and citric acid according to the prescription.
[0045] S2, dosing: Add water for injection with 70% of the prescribed amount in the dosing tank, control the temperature of the water for injection to be lower than 30°C, fill with nitrogen, add sodium citrate dihydrate, dexamethasone sodium phosphate and citric acid in sequence, Stir until completely dissolved, and add water for injection to the theoretical amount to obtain a medicinal solution.
[0046] S3, Sterilization and filtration: Filter the prepared medicinal solution through a 0.2 μm filter element to the filling production line.
[0047] S4, Filling: Use vials to fill according to th...
Embodiment 2
[0063] Based on the preparation of a 1L bottle of dexamethasone sodium phosphate injection, the dexamethasone sodium phosphate injection includes the following components in mass percentage:
[0064]
[0065]
[0066] Described preparation process comprises the steps:
[0067] S1, Weighing: Weigh dexamethasone sodium phosphate, sodium citrate dihydrate and citric acid according to the prescription.
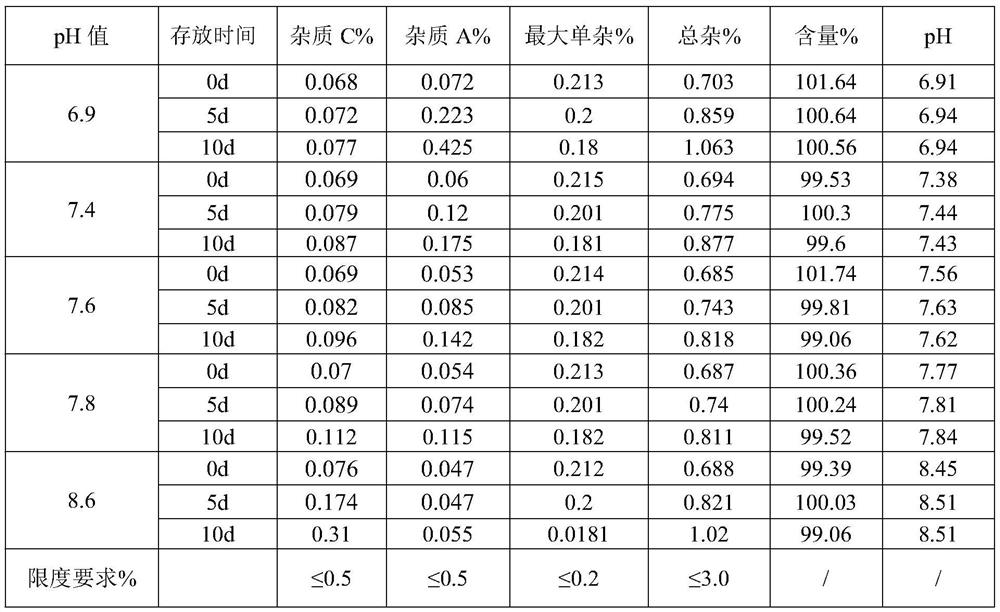
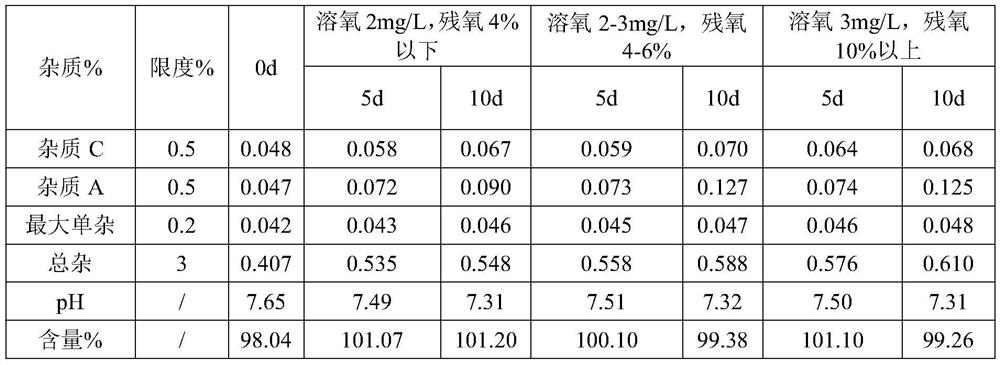
[0068] S2, dosing: Add water for injection with 70% of the prescribed amount in the dosing tank, control the temperature of the water for injection to be lower than 30°C, fill with nitrogen, add sodium citrate dihydrate, dexamethasone sodium phosphate and citric acid in sequence, Stir until completely dissolved, and add water for injection to the theoretical amount to obtain a medicinal solution. The pH value of the detected medicinal solution is 7.61, and the osmotic pressure is 286mOsm / kg.
[0069] S3, Sterilization and filtration: Filter the prepared medicinal liquid thr...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Pore diameter | aaaaa | aaaaa |
| Diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com