Antiviral drug Musellarin and analogue molecule, preparation method and application
A technology of antiviral drugs and analogs, applied in antiviral agents, organic chemistry, etc., can solve the problems of limited types of influenza virus drugs and large side effects, and achieve low cytotoxicity, low cost, and strong inhibitory effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
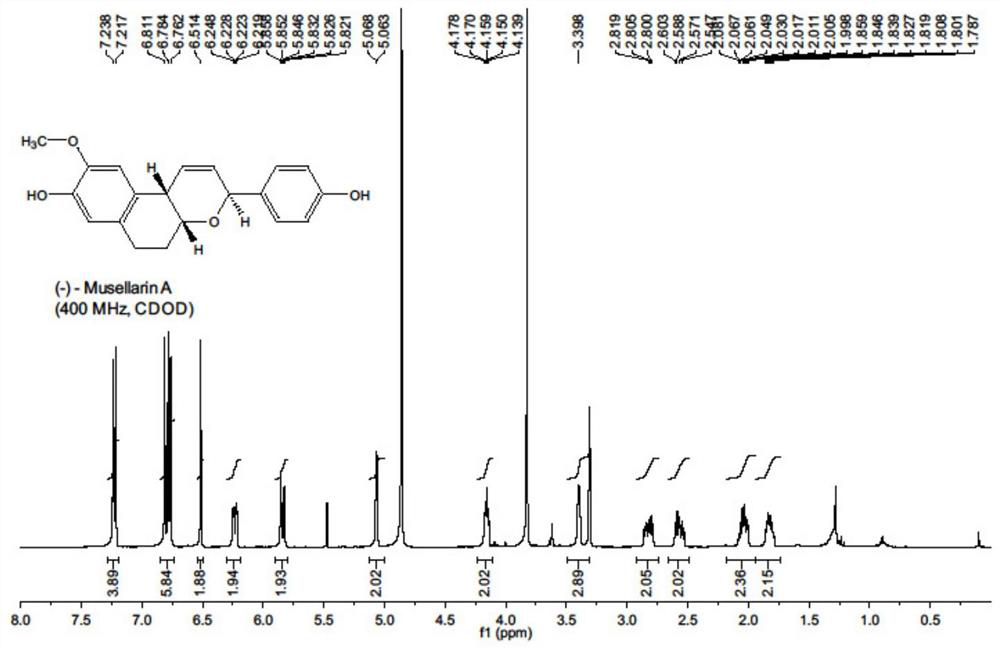
[0038] On the other hand, the embodiment of the present invention also provides a kind of antiviral drug Musellarin and the preparation method of analog molecule with influenza A virus RNA polymerase as the target, comprising the following steps:
[0039] S01: the furfuryl alcohol substrate shown in formula 4 is carried out Achmatowicz rearrangement reaction, then carries out acetylation reaction, obtains the compound shown in formula 5;
[0040] S02: deoxidizing the compound shown in formula 5 to obtain the compound shown in formula 6;
[0041] S03: The compound shown in formula 6 is carried out coupling reaction with the diazonium salt shown in formula 7-9 respectively, obtains the compound shown in formula 10-12 respectively;
[0042] S04: the compound shown in formula 10-12 is successively subjected to reduction reaction, intramolecular Friedel-Crafts ring-closure reaction and deprotection reaction;
[0043]
[0044] The embodiment of the present invention provides a m...
Embodiment 1
[0067] The preparation of compound shown in embodiment 1 formula 1-formula 3
[0068] (1) a kind of antiviral drug Musellarin and analog molecule with influenza A virus RNA polymerase as target, molecular structure is as shown in formula 1, and its preparation method comprises the following steps:
[0069] Provide 4.01g of furfuryl alcohol compound shown in Formula 4, 20ml of tetrahydrofuran / 5ml of water as the reaction medium, add 3.65g of potassium persulfate preparation, 118mg of potassium bromide and 416mg of sodium bicarbonate at 0°C, and react 1-2 at 0°C Hours, quench the reaction with sodium bicarbonate, extract and concentrate to obtain the crude product, then add the crude product to 20ml of DCM, add acetic anhydride 1.52g and triethylammonia 2.02 at 0°C, and react at 0°C for 4 hours, The reaction was quenched with aqueous ammonium chloride solution, extracted and concentrated to obtain a crude product, which was separated by column chromatography to obtain 4.1 g of d...
Embodiment 2
[0080] Embodiment 2 semi-lethal concentration (LC50) experiment
[0081] Prepare required culture media and solutions: Prepare Mirus - LT1 transfection agent and medium consisting of 5% FBS and 1% P / S Opti-MEM medium. Prepare and culture HEK-293T cells. Prepare 0.2 μg of DNA solution per well in a 96-well plate.
[0082] Cell culture: at T-75cm 2 HEK-293T cells were cultured in flasks until the cell density reached 70%-80%, and were continuously passaged for 3 times. Cells were collected 24 hours before the experiment, and each 96-well plate was inoculated with approximately 2×10 6 cells and incubate the cells overnight.
[0083] Generating transfection complex: prepare 1.43mL serum-free medium, transfect and add 22μg DNA plasmid, add 66μl TransIT-1L, mix well. Incubate at room temperature for 15-30 minutes to form transfection complexes, and add 13 μL of master mix to each well of a 96-well plate.
[0084] Trypsinize the cells in the standard medium, add an appropriat...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com