Preparation method of iguratimod intermediate
A technology of intermediates and equations, applied in the preparation of sulfonic acid amide, organic chemistry, etc., can solve the problems that sodium azide is prone to explosion, unfavorable to industrial production, and prone to side reactions, etc. Response and operation are less difficult
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
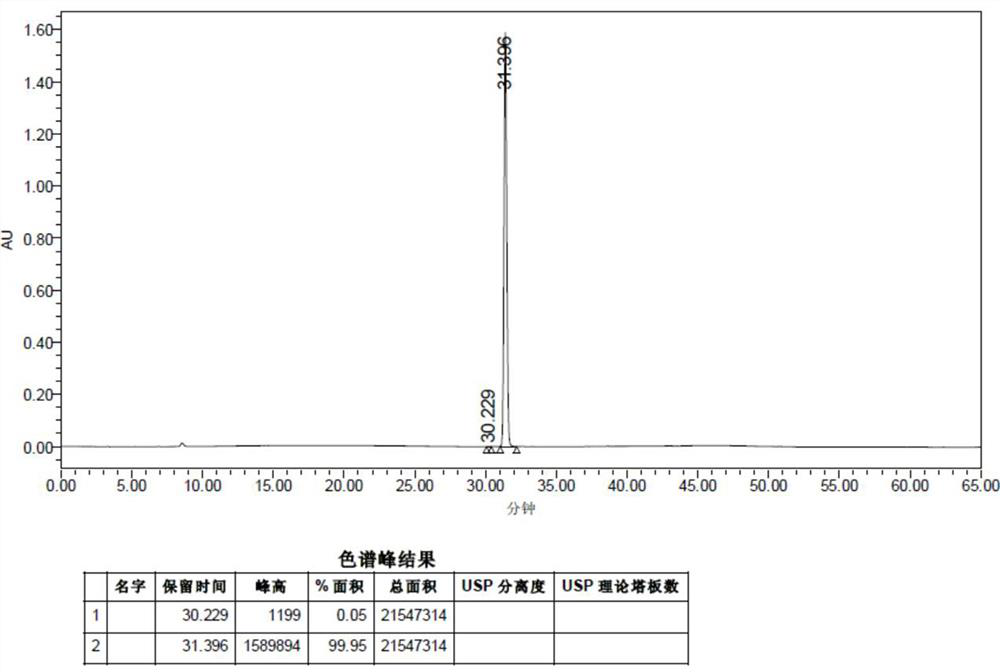
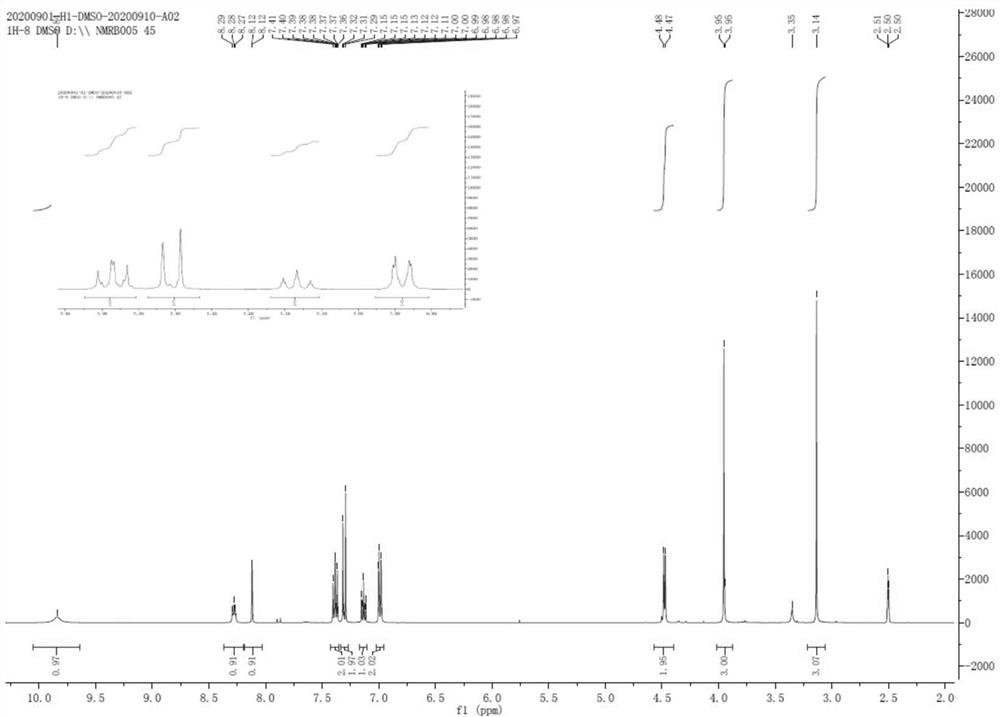
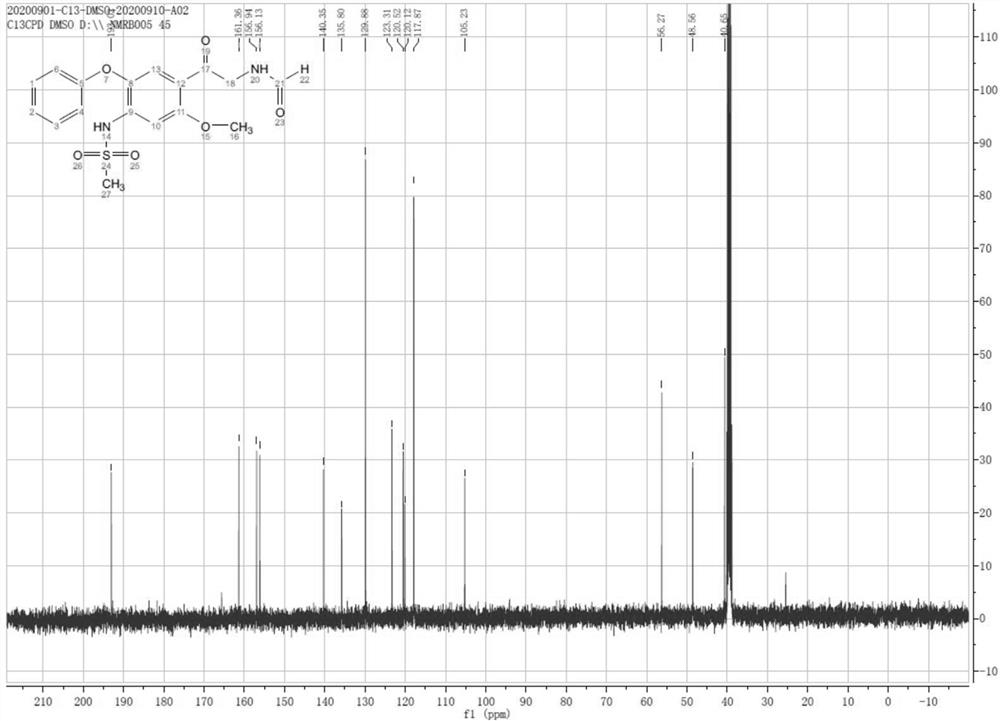
[0032] Dissolve 1.02g of formic acid in 50ml of dichloromethane, add it to a 250ml three-necked flask and stir, control the temperature at 15-25°C, add 5.28g (1.5eq) N,N-carbonyldiimidazole (CDI) in batches, and complete the addition Stir for 1 hour, the system dissolves, and add 8.40 g (1.0 eq) of 2-amino-1-(2-methoxy-4-methanesulfonamido-5-phenoxybenzene in batches at 15-25 °C Base) ethyl ketone hydrochloride (4), react for 1-2h, TLC monitors the complete reaction of the raw materials, add 50ml of purified water and 50ml of dichloromethane to the reaction system, stir for 30min, separate layers, and concentrate the organic phase to dryness under reduced pressure. Add 50ml of isopropanol to make slurry for 30min, and filter with suction to obtain compound 1 (7.54g, 91.7%) with a purity of 99.95%. 1 H-NMR (400 MHz, DMSO-d6) δ (ppm): 3.14(s, 3H), 3.95(s,3H), 4.47~4.48(d, 2H), 6.98~7.00(m, 2H), 7.11~ 7.15(m, 1H), 7.29~7.32(d,2H), 7.37~7.41(m, 2H), 8.11~8.12(d, 1H), 8.27~8.29(t,...
Embodiment 2
[0034] Dissolve 1.02g of formic acid in 50ml of dichloromethane, add it into a 250ml three-necked flask and stir, control the temperature at 15-25°C, add 3.52g (1.0eq) N,N-carbonyldiimidazole (CDI) in batches, and finish adding Stir for 1 hour, the system dissolves, and add 8.40 g (1.0 eq) of 2-amino-1-(2-methoxy-4-methanesulfonamido-5-phenoxybenzene in batches at 15-25 °C Base) ethyl ketone hydrochloride (4), react for 1-2h, TLC monitors the complete reaction of the raw materials, add 50ml of purified water and 50ml of dichloromethane to the reaction system, stir for 30min, separate layers, and concentrate the organic phase to dryness under reduced pressure. Add 50ml of isopropanol to make slurry for 30min, and filter with suction to obtain compound 1 (6.44g, 78.3%).
Embodiment 3
[0036] Dissolve 1.02g of formic acid in 50ml of dichloromethane, add it into a 250ml three-necked flask and stir, control the temperature at 15-25°C, add 7.05g (2.0eq) N,N-carbonyldiimidazole (CDI) in batches, and finish adding Stir for 1 hour, the system dissolves, and add 8.40 g (1.0 eq) of 2-amino-1-(2-methoxy-4-methanesulfonamido-5-phenoxybenzene in batches at 15-25 °C Base) ethyl ketone hydrochloride (4), react for 1-2h, TLC monitors the complete reaction of the raw materials, add 50ml of purified water and 50ml of dichloromethane to the reaction system, stir for 30min, separate layers, and concentrate the organic phase to dryness under reduced pressure. Add 50ml of isopropanol to make slurry for 30min, and filter with suction to obtain compound 1 (7.02g, 85.4%).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com