Preparation method of ceftezole acid and sodium salt thereof
A technology of ceftezole acid and tetrazolium acetic acid, which is applied in the field of medicine, can solve the problems of easy production of by-products, high equipment requirements, and increased costs, and achieve the effects of low cost, shortened reaction time, and improved yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0024] The embodiment of the present invention provides a kind of preparation method of ceftezole, comprising the following steps:
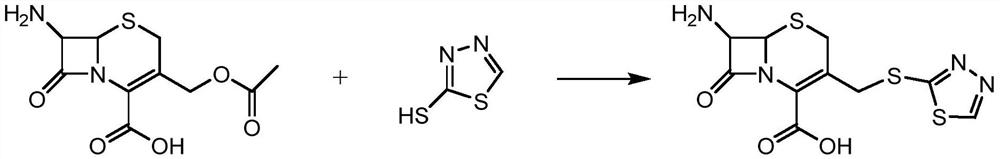
[0025] S1: 2-mercapto-1,3,4-thiadiazole and 7-ACA were used as raw materials, and boron trifluoride dimethyl carbonate complex was used as a catalyst to synthesize the intermediate 7-ACT. The involved reaction formula is as follows;
[0026]
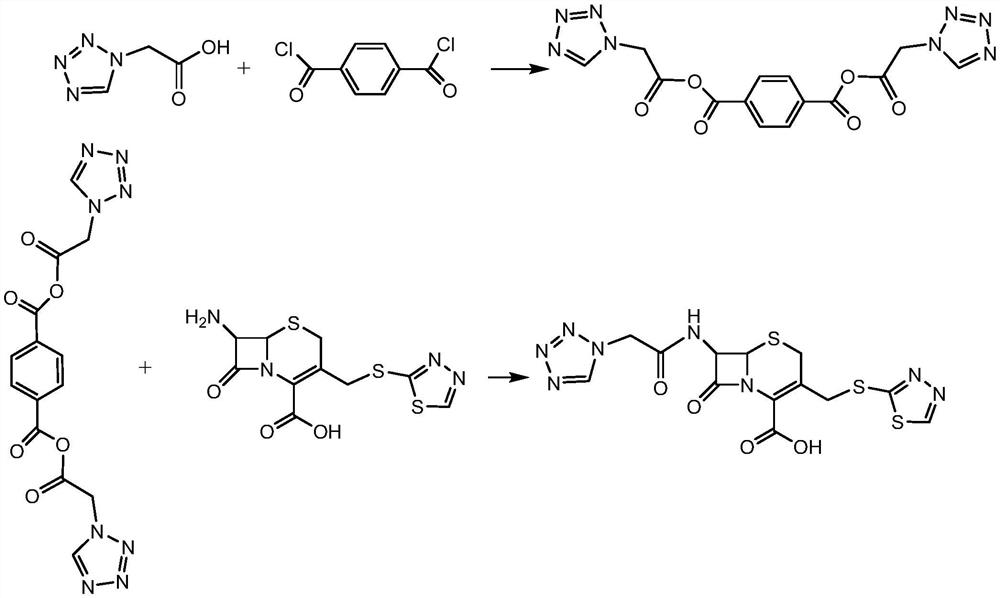
[0027] S2: Tetrazolium acetic acid is reacted with terephthaloyl dichloride to form a mixed anhydride, and then reacted with the intermediate 7-ACT to synthesize ceftezole acid. The involved reaction formula is as follows.
[0028]
Embodiment 1
[0035] A preparation method of ceftezole, comprising the steps of:
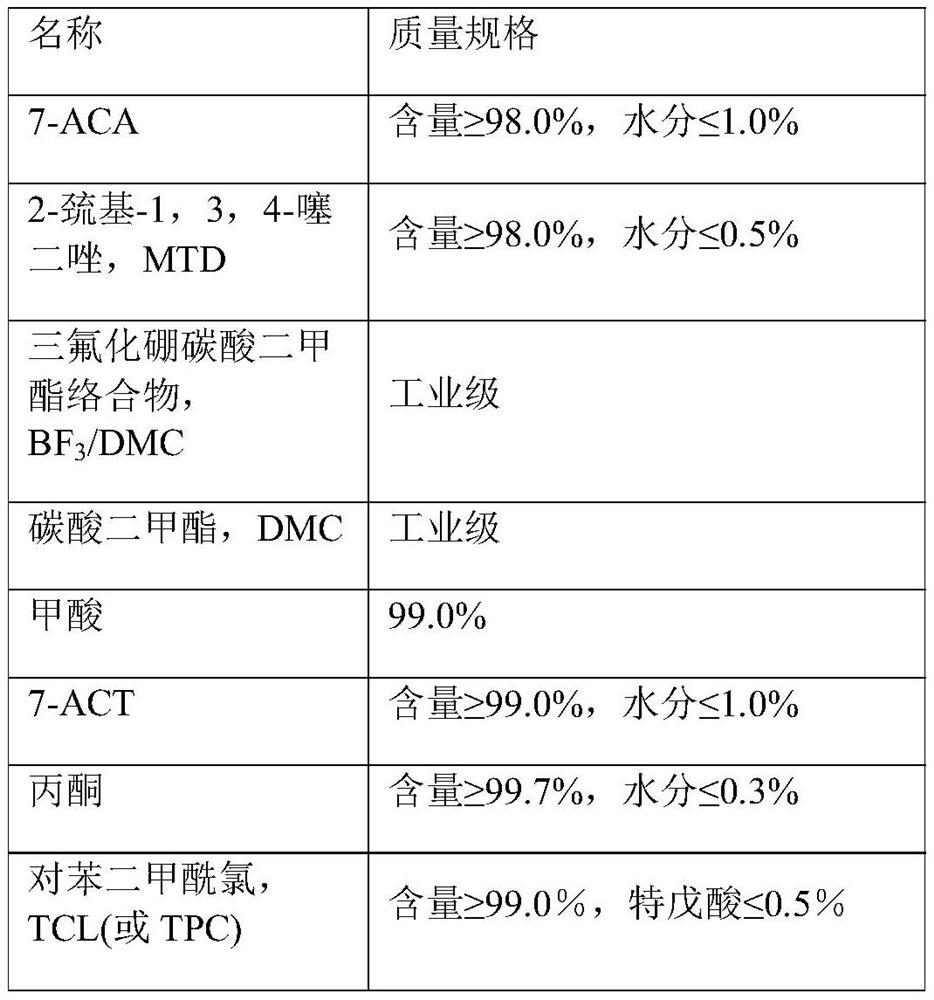
[0036] S1: Add 300L of dimethyl carbonate to the reaction kettle, under the protection of nitrogen, add 337.5kg of boron trifluoride dimethyl carbonate complex and 50kg of formic acid, stir and cool down to 4°C, under the protection of nitrogen and the temperature <10 Add 48kg of MTD and 100kg of 7-ACA under the condition of ℃, rinse the pipeline of the container with 30L dimethyl carbonate, the system starts to be turbid, and quickly becomes clear to a light yellow clear solution, and the temperature is raised to 18℃ for 35min to obtain 7-ACT The reaction solution, sampling HPLC test 7-ACA content <3%,
[0037]Add 800L of deionized water to the crystallization tank, add 0.5kg of sodium metabisulfite and 0.5kg of disodium ethylenediaminetetraacetate, stir and adjust the temperature to 4°C, add the resulting reaction solution, at 4°C Adjust the pH to 0.8 with sodium hydroxide within 70 minutes, slowly stir an...
Embodiment 2
[0044] A preparation method of ceftezole, comprising the steps of:
[0045] S1: Add 300L of dimethyl carbonate to the reaction kettle, under nitrogen protection, add 335kg of boron trifluoride dimethyl carbonate complex and 40kg of formic acid, stir and cool down to 5°C, under nitrogen protection and the temperature <10°C Adding 45kg of MTD and 100kg of 7-ACA under the same conditions, the system started to become turbid, and quickly became clear to obtain a light yellow clear solution. The temperature was raised to 20°C and reacted for 30 minutes to obtain a reaction solution containing 7-ACT. Sample HPLC was used to test the content of 7-ACA < 3%,
[0046] Add 800L of deionized water to the crystallization tank, add 0.5kg of sodium metabisulfite and 0.5kg of disodium ethylenediaminetetraacetate, stir and adjust the temperature to 5°C, add the resulting reaction solution, at 5°C Adjust the pH to 0.5 with sodium hydroxide within 60 minutes, slowly stir and grow the crystals f...
PUM
| Property | Measurement | Unit |
|---|---|---|
| purity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com