Formula and preparation process of mitochondrion oral solid preparation
A solid preparation, mitochondrial technology, which is applied in pharmaceutical formulations, pill delivery, medical preparations containing active ingredients, etc., can solve problems such as difficulty in ensuring drug quality, poor accuracy of finished product loading detection, and poor quality controllability. Achieve the effect of protecting and mitochondrial function, safety, effectiveness and quality stability, and protecting cells
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
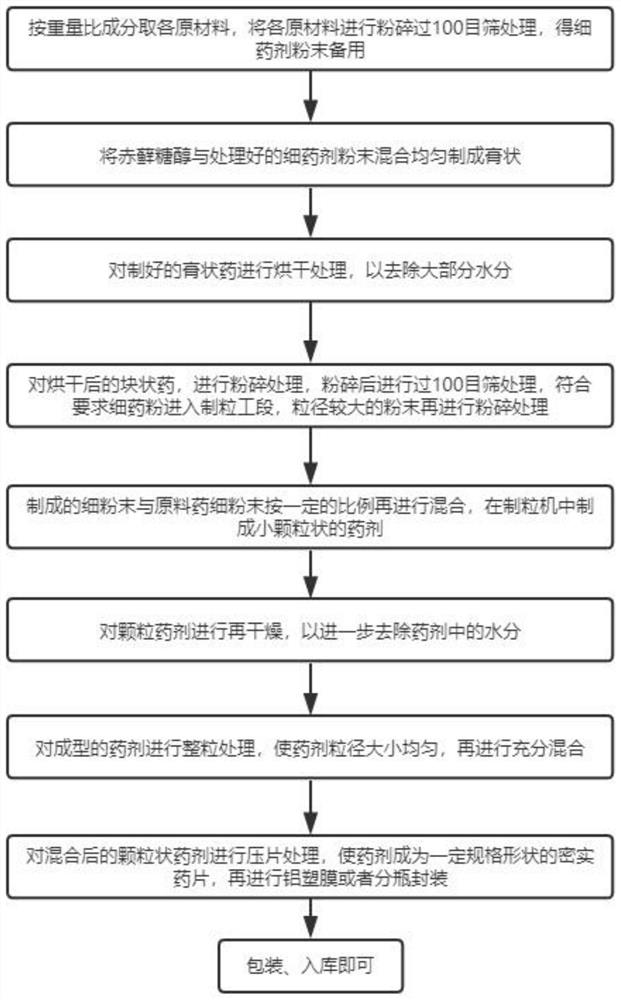
[0056] A Mitochondrial Oral Solid Preparation Formulation, such as figure 1 As shown, it includes the following components by weight: 25 parts of NADH, 30 parts of NR, 20 parts of pomegranate polyphenol, 55 parts of erythritol, 100 parts of microcrystalline cellulose, 5 parts of silicon dioxide, and the balance is water.
[0057] The preparation method of described NADH, comprises the steps:
[0058] S11: Add the same amount of boiling water to the freshly squeezed yeast under stirring, heat to 95°C and keep it warm for 5 minutes, quickly add ice cubes twice the weight of the yeast and filter, wash the filter cake twice with water, combine the filtrate and washing liquid, and add strong alkali Quaternary ammonium type I anion exchange resin 201 × 7, filtered after stirring for 16 hours, and collected the filtrate;
[0059] S12: Adjust the filtrate to PH=2-2.5 with concentrated hydrochloric acid, absorb it on a weakly acidic acrylic cation exchange resin 122 column, wash it wi...
Embodiment 2
[0099] A Mitochondrial Oral Solid Preparation Formulation, such as figure 1 As shown, it includes the following components by weight: 20 parts of NADH, 20 parts of NR, 25 parts of pomegranate polyphenol, 60 parts of erythritol, 103 parts of microcrystalline cellulose, 6 parts of silicon dioxide, and the balance is water.
[0100] The preparation method of described NADH, comprises the steps:
[0101] S11: Add the same amount of boiling water to the freshly squeezed yeast under stirring, heat to 95°C and keep it warm for 5 minutes, quickly add ice cubes twice the weight of the yeast and filter, wash the filter cake twice with water, combine the filtrate and washing liquid, and add strong alkali Quaternary ammonium type I anion exchange resin 201 × 7, filtered after stirring for 16 hours, and collected the filtrate;
[0102] S12: Adjust the filtrate to PH=2-2.5 with concentrated hydrochloric acid, absorb it on a weakly acidic acrylic cation exchange resin 122 column, wash it wi...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com