Fusion protein and application of fusion protein in degrading polymers
A technology of fusion protein and carbohydrate, applied in fusion protein and its application field in degrading polymer, can solve problems such as effect to be improved
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0041] Embodiment 1: Preparation of cutinase HiC and fusion protein HiC-TrCBM
[0042] Specific steps are as follows:
[0043] Synthetic nucleotide sequence is as shown in SEQ ID NO.14 the gene HiC of coding cutinase HiC (amino acid sequence is as shown in SEQ ID NO.1); The gene HiC of coding cutinase and pET-32b (+) plasmid pass Megaprimer PCRofWhole Plasmids (MEGAWHOP) (specific references: Miyazaki K, MEGAWHOP cloning: amethod of creating random mutagenesis via megaprimer PCR of whole plasmamids, Methods Enzymol.2011; 499:399-406) was ligated to obtain the recombinant plasmid pET-32b ( +)-HiC; transform the recombinant plasmid pET-32b(+)-HiC into E.coli BL21 to obtain the transformation product; spread the transformation product on LB solid medium (containing 100 μg·mL -1 ampicillin) and cultured upside down in a constant temperature incubator at 37°C for 8-12 hours to obtain transformants; pick the transformants and inoculate them into LB liquid medium, shake the flask at...
Embodiment 2
[0046] Example 2: Degradation of polyethylene terephthalate (PET) by HiC and HiC-TrCBM
[0047] Specific steps are as follows:
[0048] The PET film was incubated with SDS solution with a concentration of 0.1% (v / v) at 50°C for 30 minutes, then with ultra-clean water at 50°C for 5 minutes, then with absolute ethanol at 50°C for 5 minutes, and then dried at 50°C to obtain the treated PET film; respectively add cutinase HiC and fusion protein HiC-TrCBM (40U) in the embodiment 1 containing 1 * 1cm 2 In a glass test tube of PET film, degrade on a constant temperature water bath shaker at 50° C. and 150 rpm for 4 days to obtain a degradation product.
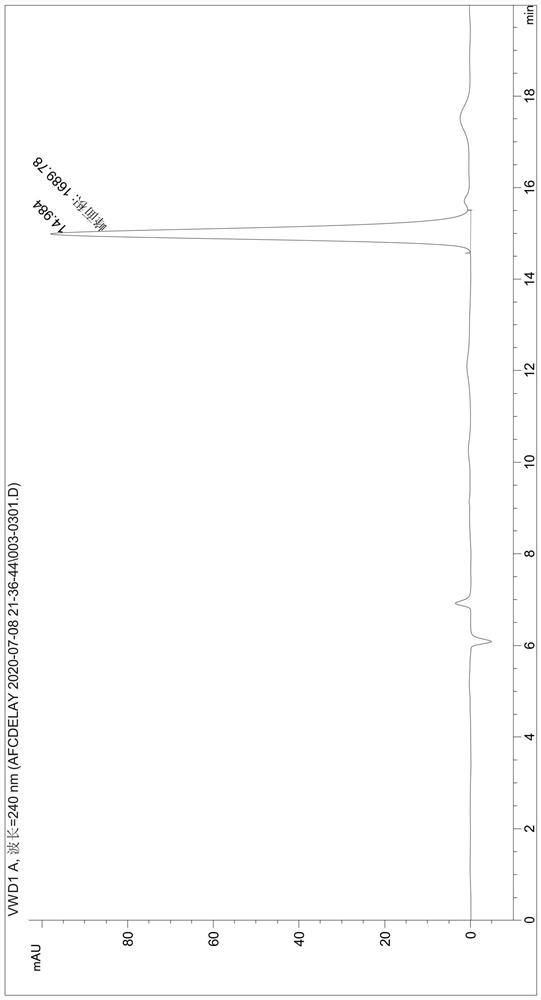
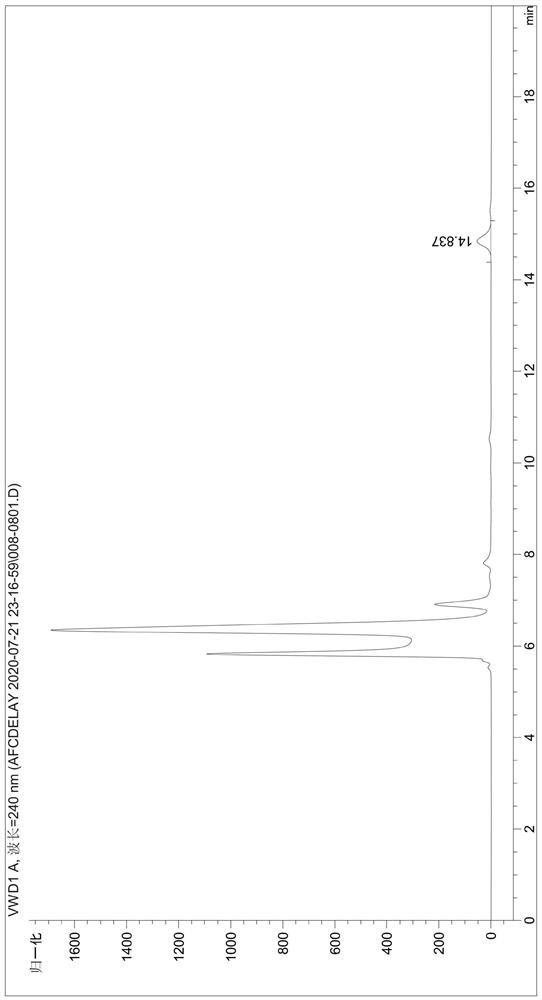
[0049] Detect the content of phthalic acid in the degradation product (the liquid phase diagram of the TPA standard is shown in figure 1 , the liquid phase diagram of the degradation product obtained by the fusion protein degraded PET film is shown in figure 2 ), the test results were: the content of TPA in the degradation produc...
Embodiment 3
[0050] Embodiment 3: Preparation of cutinase HiC and fusion protein HiC-LCI
[0051] Specific steps are as follows:
[0052] Synthetic nucleotide sequence is as the gene HiC (amino acid sequence is as shown in SEQ ID NO.1) of the coding cutinase HiC shown in SEQ ID NO.14; The gene HiC and pET-14b (+) plasmid of coding cutinase are passed Megaprimer PCRofWhole Plasmids (MEGAWHOP) (specific references: Miyazaki K, MEGAWHOP cloning: amethod of creating random mutagenesis via megaprimer PCR of whole plasmamids, Methods Enzymol.2011; 499:399-406) was ligated to obtain the recombinant plasmid pET-14b ( +)-HiC; transform the recombinant plasmid and pET-14b(+)-HiC into E.coli BL21 to obtain the transformation product; spread the transformation product on LB solid medium (containing 100 μg·mL -1 ampicillin) and cultured upside down in a constant temperature incubator at 37°C for 8-12 hours to obtain transformants; pick the transformants and inoculate them into LB liquid medium, shake ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com