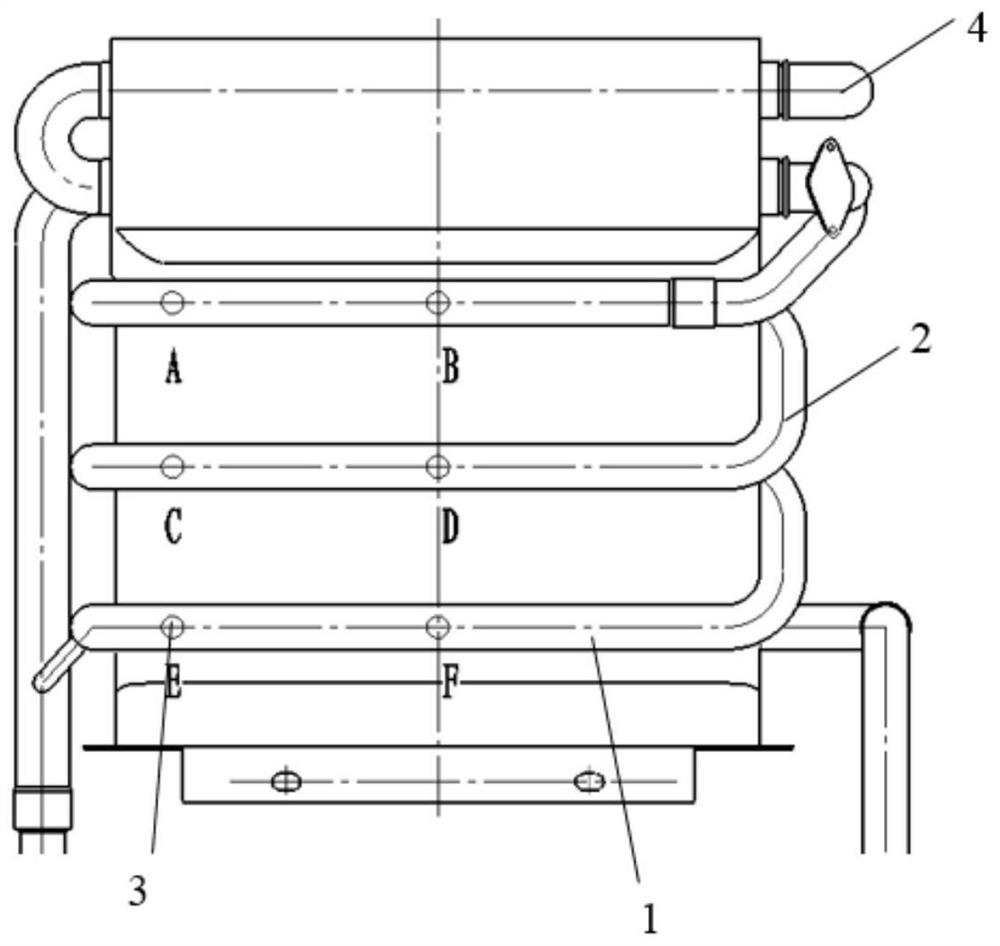
Corrosion liquid and application thereof
A technology of corrosive liquid and concentration, which is applied in the field of corrosion of metal materials, and can solve problems such as unfavorable, improved heat exchanger quality, and long cycle
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0047]The corrosion solution of this embodiment includes 200g tap water, 1.5g calcium chloride, 10g sodium chloride, 0.28g sodium bicarbonate, 6g sodium hypochlorite, and 2g sodium persulfate.
[0048] That is, the ratio of the amount of calcium chloride to sodium chloride per liter of water is 0.01:0.8.
[0049] The chloride ion concentration is 0.87mol / L, the carbonate ion concentration is 0.01mol / L, the sulfate ion concentration is 0.08mol / L, and the hypochlorite ion concentration is 0.4mol / L.
[0050] The preparation process is as follows:
[0051] Take by weighing 1.5g calcium chloride, 10g sodium chloride, 0.28g sodium bicarbonate, 6g sodium hypochlorite, 2g sodium persulfate, and set aside;
[0052] Dissolve 1.5g of calcium chloride, 10g of sodium chloride, 0.28g of sodium bicarbonate, 6g of sodium hypochlorite, and 2g of sodium persulfate in 200g of tap water, stir, and wait for complete dissolution to obtain the corrosion solution of this embodiment.
[0053] When t...
Embodiment 2
[0057] The corrosion solution of this embodiment includes 200g tap water, 1.5g calcium chloride, 10g sodium chloride, 0.28g sodium bicarbonate, 6g sodium hypochlorite, and 0.5g sodium persulfate.
[0058] That is, the ratio of the amount of calcium chloride to sodium chloride per liter of water is 0.01:0.8.
[0059] The chloride ion concentration is 0.87mol / L, the carbonate ion concentration is 0.01mol / L, the sulfate ion concentration is 0.04mol / L, and the hypochlorite ion concentration is 0.4mol / L.
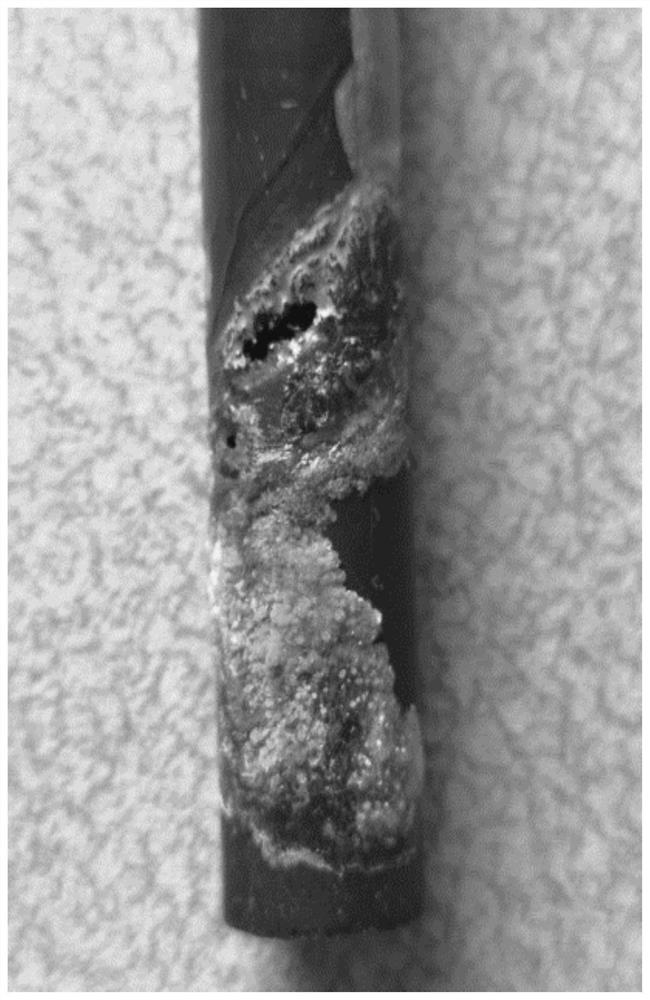
[0060] When the corrosion solution of this embodiment is circulated through the heat exchanger made of red copper, corrosion perforation occurs after 25 days, and the results are as follows: image 3 shown. By analyzing the corrosion results of this embodiment, it is found that:
[0061] The corrosion direction is from the inside (inner wall) to the outside (outer wall). Internal corrosion products are basic copper carbonate and / or copper hydroxide. The external corrosion pro...
Embodiment 3
[0064] The corrosion solution of this embodiment includes 1000g tap water, 1.5g calcium chloride, 10g sodium chloride, 0.28g sodium bicarbonate, 6g sodium hypochlorite, and 2g magnesium sulfate.
[0065] That is, the ratio of the amount of calcium chloride to sodium chloride per liter of water is 0.01:0.8.
[0066] The chloride ion concentration is 0.87mol / L, the carbonate ion concentration is 0.01mol / L, the sulfate ion concentration is 0.08mol / L, and the hypochlorite ion concentration is 0.4mol / L.
[0067] When the corrosive liquid of this embodiment was circulated through the heat exchanger composed of TP2, corrosion perforation appeared after 20 days. By analyzing the corrosion results of this embodiment, it was found that:
[0068] The corrosion direction is from the inside (inner wall) to the outside (outer wall). Internal corrosion products are basic copper carbonate and / or copper hydroxide. The external corrosion products are copper sulfate and copper chloride, and th...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com