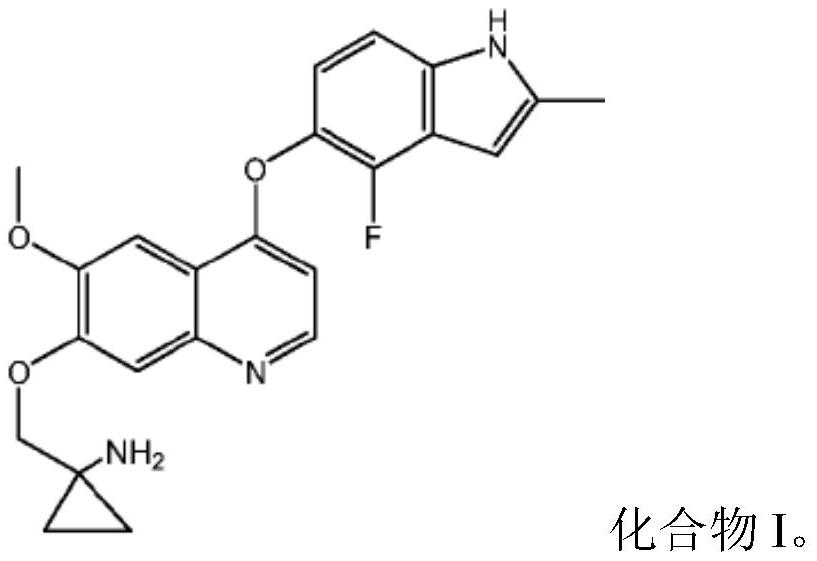
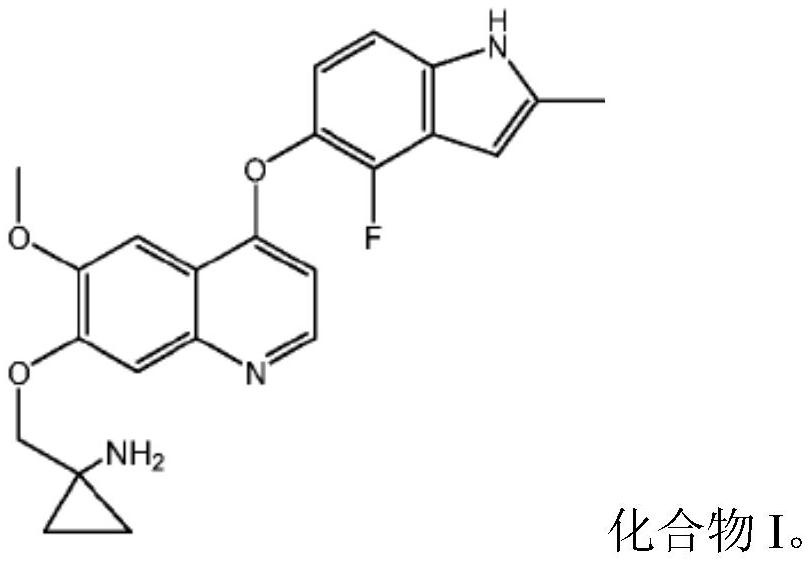
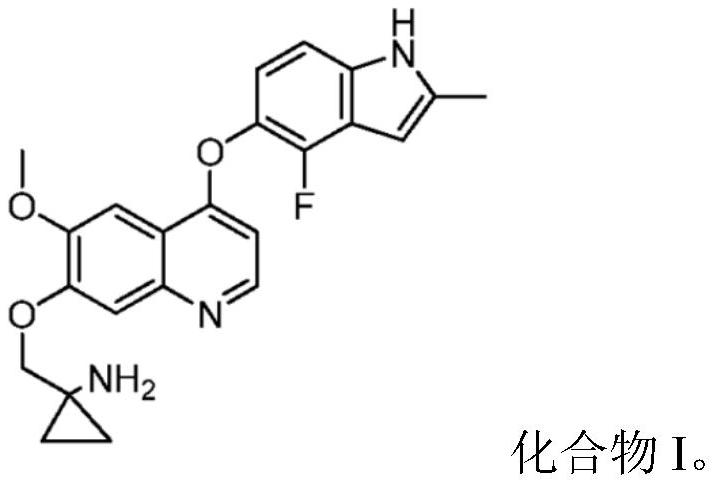
Quinoline compound for combined treatment of esophageal cancer
A technology for esophageal cancer and compounds, applied in the field of pharmaceutical preparations and medicine, can solve the problems of cell vacuolation, nausea, and lumen expansion
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0137] Esophageal squamous cell carcinoma confirmed by histology or cytology, and patients with locally advanced unresectable, local recurrence or distant metastasis; the commonly used method of block randomization is divided into group A and group B, respectively combined with drug therapy Rotinib and irinotecan, and irinotecan alone, the specific dosage regimen is as follows:
[0138] Group A (test group):
[0139] Anlotinib hydrochloride combined with irinotecan: Take anlotinib hydrochloride capsules 12mg (specification: 12mg / capsule) on an empty stomach every day, orally for 2 weeks and stop for 1 week, irinotecan 65mg / m 2 , d1, d8 intravenous infusion. 3 weeks as a treatment cycle, until disease progression (PD) or toxicity intolerable.
[0140] Group B (control group):
[0141] Irinotecan 65mg / m 2 , d1, d8 intravenous infusion. 3 weeks as a treatment cycle, until disease progression (PD) or toxicity intolerable.
[0142] The general description of the patients is s...
Embodiment 2
[0152] Patients with locoregional recurrence of esophageal squamous cell carcinoma confirmed by histopathology and / or cytology examination, radiotherapy combined with anlotinib, the specific administration is as follows:
[0153] Radiation therapy: 59.4Gy / 1.8Gy / 33Fx; anlotinib hydrochloride capsules: 12mg po d1-14 on day 1 to day 14 of each cycle, every 3 weeks as a treatment cycle.
[0154] The observed efficacy indicators include objective response rate (ORR), treatment-related toxicity, local control rate (LCR), distant metastasis rate (DMR), progression-free survival (PFS), and overall survival (OS).
[0155] This study shows that for patients with esophageal cancer, the treatment regimen of anlotinib combined with radiotherapy has clinical benefits.
Embodiment 3
[0157] Potentially resectable stage IIB-IVA esophageal squamous cell carcinoma confirmed by histopathology or cytology, with at least one measurable lesion after treatment (helical CT scan long diameter ≥ 10 mm, meeting the requirements of RESCIST version 1.1 criteria) The patient was given anlotinib, paclitaxel liposome and nedaplatin in combination for preoperative neoadjuvant therapy, and the specific dosage regimen was as follows:
[0158] Anlotinib Hydrochloride Capsules: Take it on an empty stomach before breakfast, once a day, 1 capsule (12mg) each time, take 2 weeks in a row, every 3 weeks as a cycle, preoperative administration for 2 cycles, postoperative administration for 4 -6 cycles, then anlotinib maintenance therapy until progression;
[0159] Paclitaxel liposome: 175mg / m 2 , intravenous infusion on the first day of each cycle, every 3 weeks as a cycle, 2 cycles of preoperative administration, and 4-6 cycles of postoperative administration;
[0160] Nedaplatin:...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com