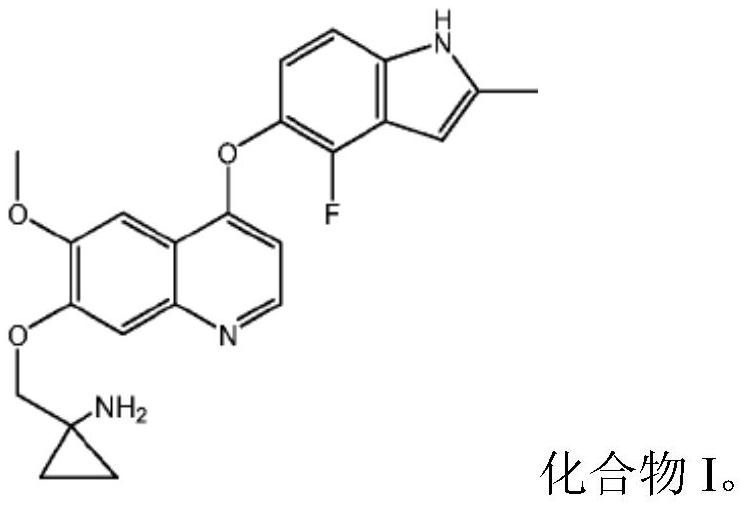
Quinoline compound for combined treatment on carcinoma of colon and rectum
A colorectal cancer and compound technology, applied in the field of pharmaceutical preparations and medicine, can solve problems such as strong side effects and lack of more effective therapeutic drugs
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0206] Example 1 Phase I / II exploratory study of anlotinib combined with irinotecan in the second-line treatment of mCRC
[0207] In patients with histologically and / or cytologically confirmed advanced metastatic colorectal cancer, combined administration of anlotinib and irinotecan, anlotinib once a day (qd) for 2 weeks and rest for 1 week, combined Irinotecan 180mg / m 2 , injected once every two weeks in each cycle, every 42 days as a treatment cycle, collecting safety information until disease progression or toxicity intolerable, so as to determine the efficacy of anlotinib combined with irinotecan in patients with advanced colorectal cancer The maximum tolerated dose (MTD, Maximum Tolerated Dose) and / or the recommended phase II dose (RP2D, Recommended Phase II Dose). The specific dosage regimen has the following three dosage groups from low to high:
[0208] A: Anlotinib 8mg qd, take two weeks and take one week off, + irinotecan 180mg / m 2 , injected once every two weeks;...
Embodiment 2
[0214] Example 2 Anlotinib hydrochloride combined with CAPEOX regimen for first-line treatment of RAS / BRAF wild-type advanced mCRC
[0215] Patients with stage IV (T1-4N0-2M1) unresectable or potentially resectable locally advanced, locally recurrent or metastatic colorectal cancer confirmed by histopathology, in which the primary tumor or metastatic tumor is detected as RAS / BRAF wild-type patients who have not previously received systemic therapy, including chemotherapy, targeted and immunotherapy, or (neo) adjuvant chemoradiotherapy / radical surgery for more than 6 months of recurrence, combined administration of anlotinib and CAPEOX Program. The specific administration method is as follows, and the following medicines all adopt 3 weeks (21 days) as a treatment cycle:
[0216] Anlotinib hydrochloride 12mg, orally once a day, on days 1-14 of each cycle;
[0217] Oxaliplatin 130mg / m 2 , intravenous drip, medication on the first day of each cycle;
[0218] Capecitabine 1000...
Embodiment 3
[0222] Example 3 First-line treatment of advanced mCRC with anlotinib hydrochloride combined with CAPEOX regimen
[0223] In patients with histologically or cytologically confirmed colon or rectal adenocarcinoma, combined administration of anlotinib and CAPEOX regimen.
[0224] The specific administration method is as follows:
[0225]
[0226] Among them, at least 6 cycles of initial treatment, capecitabine: 1000mg / m 2 , calculated based on the patient's body surface area at baseline.
[0227] To evaluate the efficacy and safety of anlotinib combined with CAPEOX regimen in the first-line and maintenance treatment of patients with metastatic colorectal cancer. The observed indicators are mainly progression-free survival (PFS), objective response rate (ORR), duration of response ( DOR), disease control rate (DCR) and safety indicators.
[0228] Clinical trial results: 3 patients have been evaluated so far, of which 1 patient achieved PR (Partial Response), and 2 patients ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com