Novel coronavirus neutralizing antibody detection kit
A coronavirus and kit technology, applied in the field of cell biology and immunoassay, can solve the problems of inability to comprehensively evaluate the activity of neutralizing antibodies, high experimental safety level requirements, and high experimental operation requirements, achieving short detection time and overcoming the safety level The effect of high requirements and low laboratory requirements
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
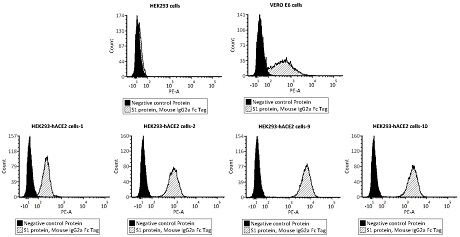
[0038] Example 1 Preparation and identification of HEK293-hACE2 cells
[0039] 1. Experimental materials
[0040] 1. Main reagents:
[0041] HEK293 cells (Thermofisher, Cat. No. A14528 ); puromycin (Sangon Bioengineering (Shanghai) Co., Ltd., Cat. No. PJ593-25mg); fetal bovine serum FBS (CellMax, Cat. No. SA212.02 ); DMEM medium (Hyclone, Cat. No. SH30022.01); 0.25% trypsin (0.25% Trypsin-EDTA, Gibco, Cat. No. 25200-056); linear polyethyleneimine (Polyethyleneimine, Linear, Polysciences, Cat. No. 23966); PB-hACE2 expression vector (provided by Acrobiosystems molecular construction department); trypan blue staining solution (Trypan Blue Solution, 0.4%, Thermofisher, Cat. No. 15250061); new coronavirus spike sugar Protein S1 (SARS-CoV-2 (COVID-19) S1 protein, Mouse IgG2a Fc Tag, Acrobiosystems, Cat. No. S1N-C5257); Negative control protein (Human Epidermal Growth Factor Receptor 2 Protein Human Her2 Protein, Mouse IgG2a Fc Tag, Acrobiosystems, Cat. No. HE2-H5255); PE-labeled ...
Embodiment 2
[0057] Example 2 Preparation and identification of PE-labeled coronavirus spike glycoprotein S1
[0058] 1. Experimental materials
[0059] 1. Main reagents:
[0060] Biotinylated SARS-CoV-2 (COVID-19) S1protein, His, Avitag™ (MALS verified), Acrobiosystems, Cat. No. S1N-C82E8); PE-labeled streptavidin Avidin (PE-Streptavidin, Jackson, Cat. No. 016-110-084); Bovine Serum Albumin (Bovine Serum Albumin, BSA, Sigma-Aldrich, Cat. No. SRE0098-100G); D-(+) - Trehalose dihydrate (Sigma-Aldrich, Cat. No. T9531-10G); Phosphate buffered saline (PBS buffer, Hyclone, Cat. No. SH30256.01).
[0061] 2. Main consumables and instruments:
[0062] Cell culture dish (Thermo Fisher Scientific, Cat. No. 150466); 15ml sterile centrifuge tube (TrueLine, Cat.No. TR2010); 50ml sterile centrifuge tube (TrueLine, Cat.No. TR2012); pipette (Rui Ning RAININ); vials (Short Xinkang Pharmaceutical Packaging Co., Ltd., Boron 260); freeze dryer (Telstar, lyobeta5PS); vortex mixer (Jiangsu Kangjian Medical ...
Embodiment 3
[0078] Example 3 Establishment of flow cytometric detection method for neutralizing antibodies against novel coronavirus
[0079] 1. Experimental materials
[0080] PE-labeled SARS-CoV-2 spike glycoprotein S1; HEK293-hACE2 cells; SARS-CoV-2 neutralizing antibody standard, Anti-SARS-CoV-2 RBD Neutralizing Antibody, Human IgG1 (Acrobiosystems, Cat.No. SAD-S35) ; negative control antibody sample, Human IgG1 (N297A) Isotype Control (Acrobiosystems, Cat. No. DNP-MB273); normal human serum (Abbkine, Cat. No. BMS0060); flow detection buffer; PBS buffer.
[0081] Ultra-clean bench (Beijing Donglian Haer Instrument Manufacturing Co., Ltd., Cat. No. DL-CJ-2ND). Carbon dioxide incubator (Thermo Fisher Scientific, Thermo 3111); LUNA II automatic counter (LUNA, LunaⅡ); Xiangyi low-speed centrifuge (Xiangyi Centrifuge Instrument Co., Ltd., L-550); Inverted microscope (OLYMPUS, CKX53) ; flow cytometer (BD FACSCelesta™ flow cytometer).
[0082] 2. Establishment of a flow cytometric detecti...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com