Method for detecting target miRNA based on G-quadruplex molecular beacon double-enzyme cascade isothermal amplification
A purpose and polymerase technology, applied in the field of biomedicine, can solve the problem that a single miRNA cannot provide clear results for early cancer diagnosis and treatment monitoring, and achieve high accuracy, good specificity, and high sensitivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
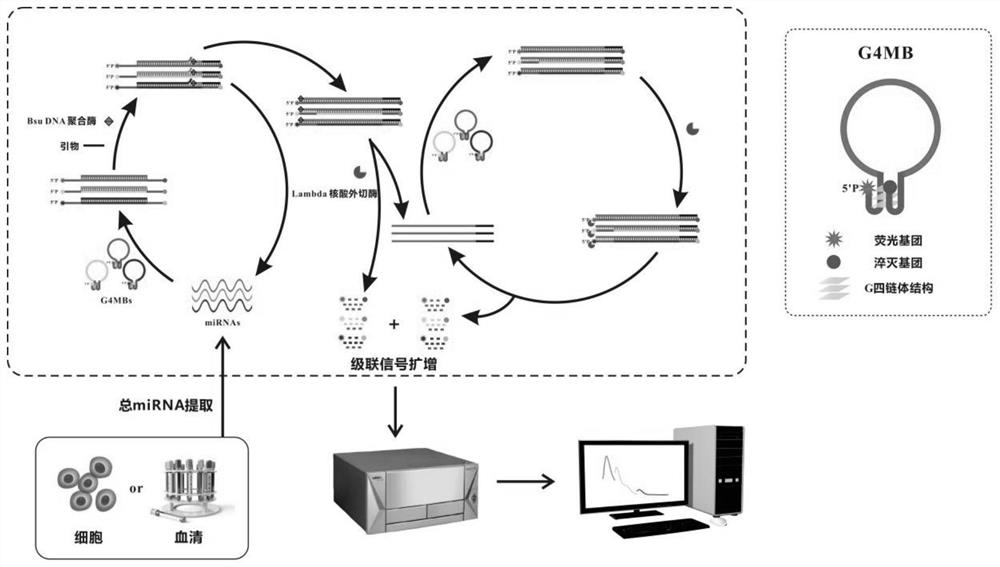
[0069] Example 1. Establishment of a method for detecting miRNA using G quadruplex molecular beacon (G4MB) dual-enzyme cascade isothermal amplification
[0070] After a lot of experiments, the inventors of the present invention have established a method for detecting miRNA using G4MB double-enzyme cascade isothermal amplification. The chain body structure is released, and the primer has the opportunity to combine with the primer at the 3' end of G4MB; under the action of the primer and Bsu DNA polymerase, a double-stranded hybrid structure is synthesized using the opened G4MB as a template, and replaces the original target miRNA; The substituted miRNA can bind to other G4MBs and participate in the next cycle. In this cycle, a single miRNA can trigger the restoration of multiple G4MBs signaling, achieving a round of amplification of miRNA signaling. At the same time, once Bsu DNA polymerase catalyzes the synthesis of hybrid double strands, Lambda exonuclease can gradually dige...
Embodiment 2
[0088] The feasibility analysis of the method that embodiment 2, embodiment 1 establish
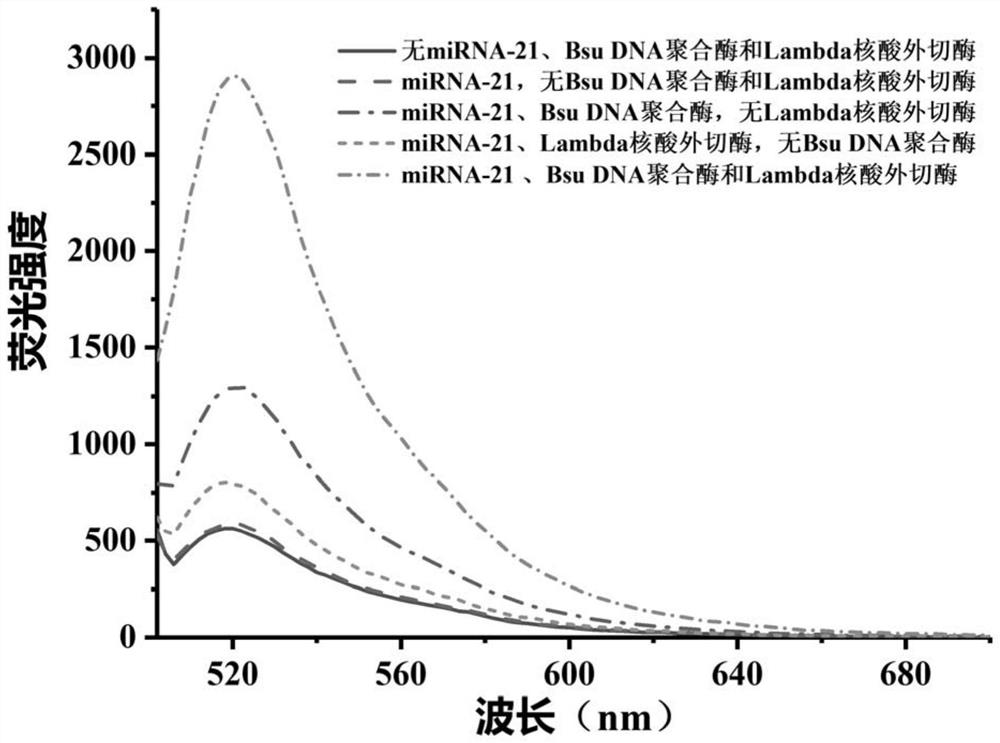
[0089] In this example, G4MB-21 was selected as the model probe, and the feasibility of detecting the target miRNA-21 by the biosensor was explored according to the method established in Example 1.
[0090] The fluorescence emission spectra of the miRNA detection system were obtained under different conditions according to the method established in Example 1 (in step 3 specifically: incubate at 37° C. for 30 min).
[0091] Condition a: The reaction system is 100uL, which is composed of reaction buffer and G4MB-21; the concentration of G4MB-21 in the reaction system is 100nM.
[0092] Condition b: the reaction system is 100uL, which is composed of reaction buffer, G4MB-21 and miRNA-21; in the reaction system, the concentration of G4MB-21 is 100nM, and the concentration of miRNA-21 is 10nM.
[0093] Condition c: The reaction system is 100uL, consisting of reaction buffer, G4MB-21, miRNA-21...
Embodiment 3
[0098] Embodiment 3, sensitivity analysis
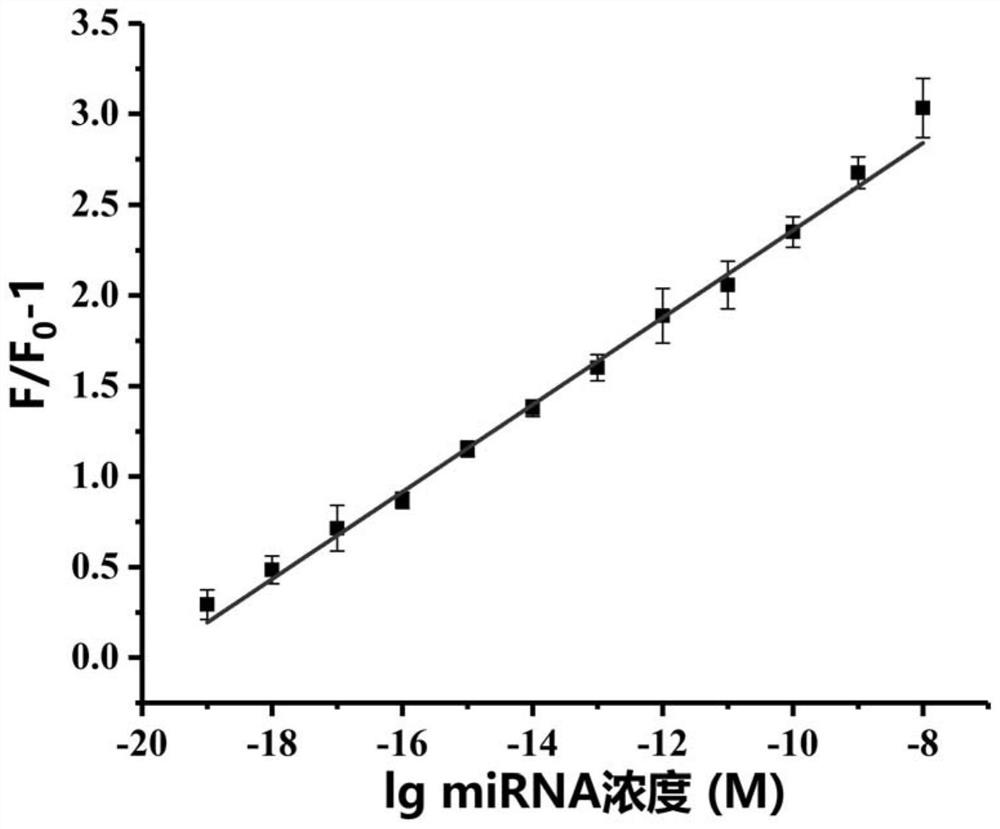
[0099] The fluorescence emission spectrum of the miRNA detection system was obtained under the reaction system according to the method established in Example 1 (in step 3 specifically: incubate at 37° C. for 30 min). The reaction system is 100uL, consisting of reaction buffer, G4MB-21, miRNA-21, Bsu DNA polymerase (5U), Lambda exonuclease (10U) and ribonuclease inhibitor (40U); in the reaction system, The concentration of G4MB-21 was 100 nM, and the concentration of miRNA-21 was 0 zM, 100 zM, 1 aM, 10 aM, 100 aM, 1 pM, 10 pM, 100 pM, 1 nM or 10 nM.
[0100] The relationship between the concentration of miRNA-21 and the fluorescence intensity is shown in image 3 (F 0 is the fluorescence intensity of the system in the absence of miRNA-21, F is the fluorescence intensity of the system in the presence of miRNA-21). The results showed that as the concentration of miRNA-21 increased from 0zM to 100nM, the fluorescence intensity also gr...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com