Single-cell Raman clinical drug resistance kit and detection method
A technology of Raman drug resistance and kits, which is applied in Raman scattering, biochemical equipment and methods, and measurement/testing of microorganisms, can solve the problems of slow detection of clinical drug-resistant pathogens, and achieve high accuracy, Simple operation and high sensitivity effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0031] Embodiment 1 kit composition information
[0032] The information of the kit in this embodiment is shown in Table 1. The incubation solution is 100% heavy water, the fixative solution I is 0.9% normal saline, the fixative solution II is absolute ethanol, and the ultrapure water and culture medium can be prepared by yourself. .
[0033] Table 1. Kit composition information
[0034]
[0035]
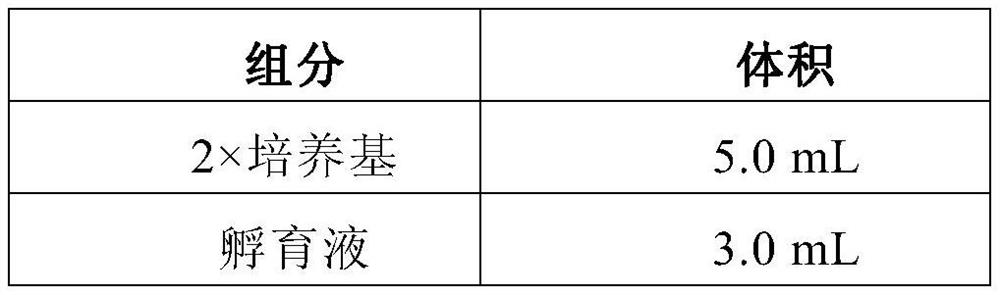
[0036] Table 2 shows the formula of the incubation system prepared when using the above-mentioned kits for rapid detection of single-cell Raman clinical drug resistance. The volume of the test drug is adjusted according to different samples.
[0037] Table 2. Preparation of incubation system
[0038] components volume 2×Medium (2×Medium) 5.0mL Incubation Buffer 3.0mL Clinical samples to be tested 1.0mL Test Drugs (Antibiotics) n mL ultrapure waterddH 2 o
Replenish to 10.0mL Total 10.0mL
[0039] Table 3 shows the...
Embodiment 2
[0042] Example 2 Detection of drug resistance of Escherichia coli (E.coli395) in clinical urine samples by single-cell Raman clinical drug resistance quick test kit
[0043] In this example, the single-cell Raman clinical drug resistance quick detection kit is used to detect the drug resistance of Escherichia coli (E.coli395) in clinical urine samples of patients with urinary tract infection, and the corresponding test drug (antibiotic) is Levofloxacin (Levofloxacin, Levo), the specific drug resistance detection steps are as follows:
[0044] 1. Clinical urine sample and drug pretreatment
[0045] Collect 1.0mL clinical fresh urine sample, centrifuge (8000rpm, 2min), remove the supernatant, add 1.0mL PBS to resuspend for use.
[0046] 2. Preparation of incubation system
[0047] Carry out the establishment of the incubation system of control group and experimental group according to Table 4, do not add one group of levofloxacin (Levo) as control group (with ultrapure water d...
Embodiment 3
[0062] Example 3 Detection of drug resistance of Escherichia coli (E.coli865) in clinical urine samples by single-cell Raman clinical drug resistance quick test kit
[0063] In this example, the single-cell Raman clinical drug resistance quick detection kit is used to detect the drug resistance of Escherichia coli (E.coli865) in clinical urine samples of patients with urinary tract infection, and the corresponding test drug (antibiotic) is Gentamicin Gentamicin (Gentamicin, Genta), the specific drug resistance detection steps are as follows:
[0064] 1. Clinical urine sample and drug pretreatment
[0065] Collect 1.0mL clinical fresh urine sample, centrifuge (8000rpm, 2min), remove the supernatant, add 1.0mL PBS to resuspend for use.
[0066] 2. Preparation of incubation system
[0067] According to Table 5, the establishment of the incubation system of the control group and the experimental group was carried out, and the group without gentamicin (Genta) was used as the cont...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com