Method for enhancing hematopoietic stem cell transplantation capacity
A technology of hematopoietic stem cells and ability, applied in the field of hematopoietic stem cells, can solve the problems of lack of HSC expansion scheme and low efficiency of HSC expansion
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0026] Example 1 Separation of CD34 from umbilical cord blood + cell
[0027] 1) Collection of cord blood
[0028] The blood from the fetal umbilical cord was collected in a sterile environment in the operating room, stored in a blood bag with anticoagulant, temporarily stored in a microenvironment at 4°C, and delivered to the laboratory for use within 24 hours.
[0029] 2) Isolation of monocytes from cord blood
[0030] a) In a sterile laboratory bench, transfer the umbilical cord blood into a sterile culture bottle, add the corresponding volume of PBS according to the volume ratio of blood: phosphate buffer solution = 1:2, and mix well.
[0031] b) Slowly add the diluted umbilical cord blood into a 50ml centrifuge tube filled with 15ml of human lymphatic separation fluid. Be careful to add slowly to keep the interface between the two liquids clear and not to break the liquid level balance between blood and lymphatic separation fluid.
[0032] c) Centrifuge at 1500 rpm...
Embodiment 2
[0051] Example 2 CD34 + Cell culture and detection
[0052] experimental method:
[0053]a) Use 50-200ng / ml SCF (100ng / ml is preferred in this example), 50-200ng / ml Flt-3L (100ng / ml is preferred in this example), 10-100ng / ml TPO (50ng / ml is preferred in this example), 1 Resuspend CD34 in StemSpan medium with ~100μg / ml LDL (preferably 50μg / ml in this example) + For cells, add 8-20 μM (preferably 15 μM in this example) SP600125 / JNK-IN-8 / DMSO, add it to a 6-well low-attachment plate, and control the cell density not higher than 1×10 6 tablets / ml;
[0054] b) Place the cells at 37°C, 5% CO 2 cultured in a cell culture incubator;
[0055] c) Every 2 days, replace half of the medium to ensure a cell density of 1×10 6 grains / ml or less.
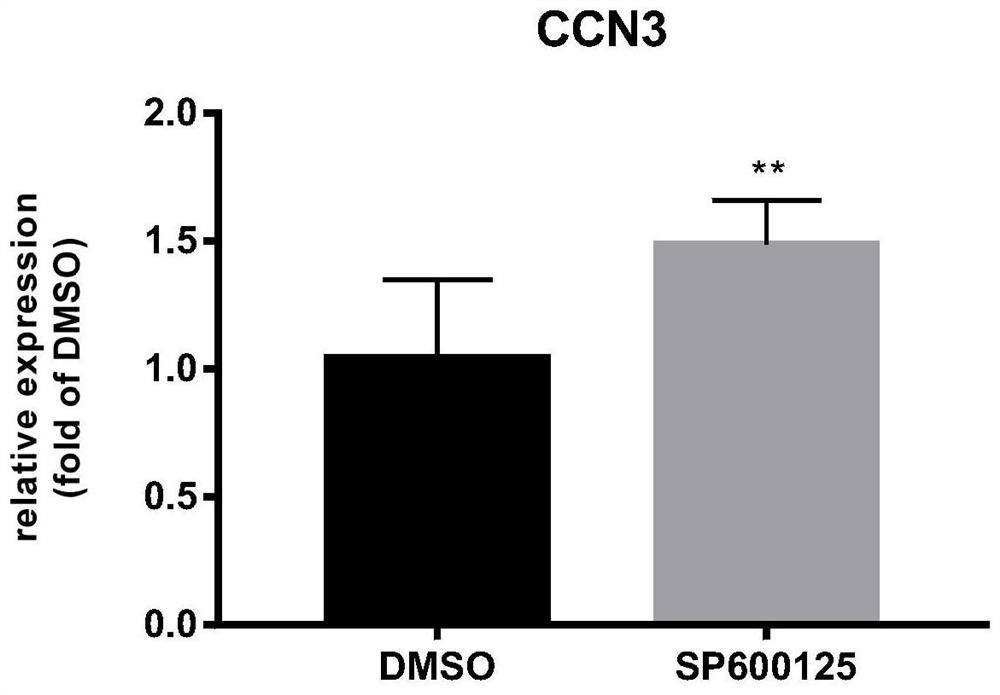
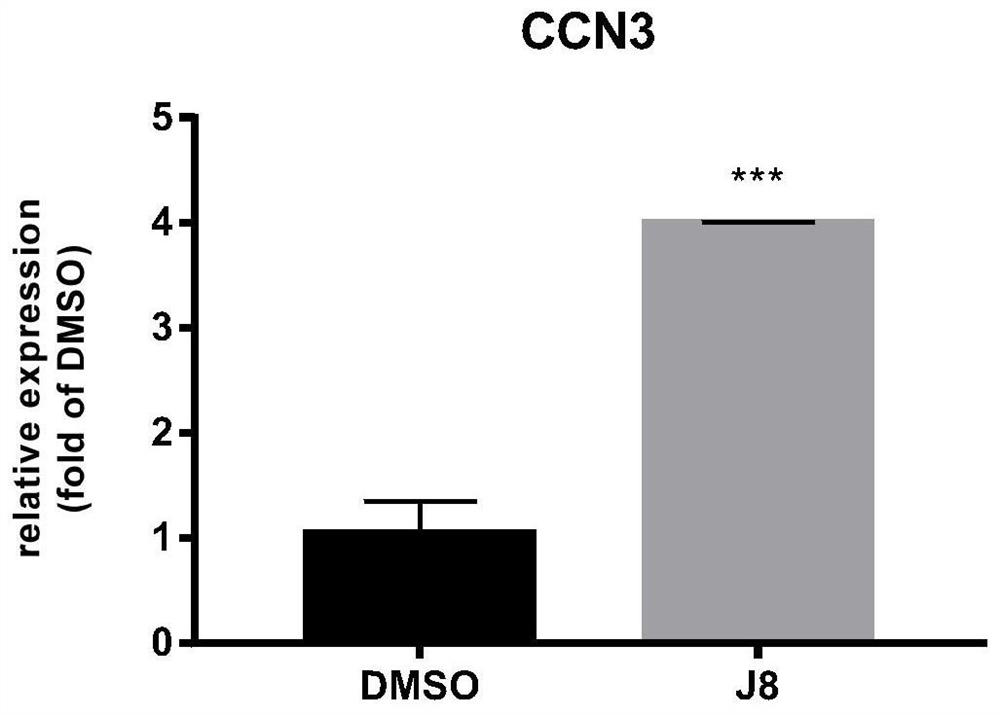
[0056] Cells were cultured for 48 hours, cellular RNA was extracted (QIAGEN RNAEASY PLUS MINI KIT cat:74136), RNA was reverse transcribed into cDNA, and qPCR experiments were carried out using cDNA as a template. The result is as figure 1 ,...
Embodiment 3
[0058] Embodiment 3CFU colony formation experiment
[0059] experimental method:
[0060] 1) Inoculate the cells obtained in Example 2 for 7 days into 2ml of methylcellulose, and mix well;
[0061] 2) Use a 1ml syringe to transfer the methylcellulose mixed with the cell suspension (about 10,000 cells) to a low-attachment 6-well plate;
[0062] 3) Place at 37°C, 5% CO 2 Cultivate in the concentration incubator for 14 days;
[0063] 4) Observe the number of different cell colonies formed under a microscope, and make a record.
[0064] Experimental results:
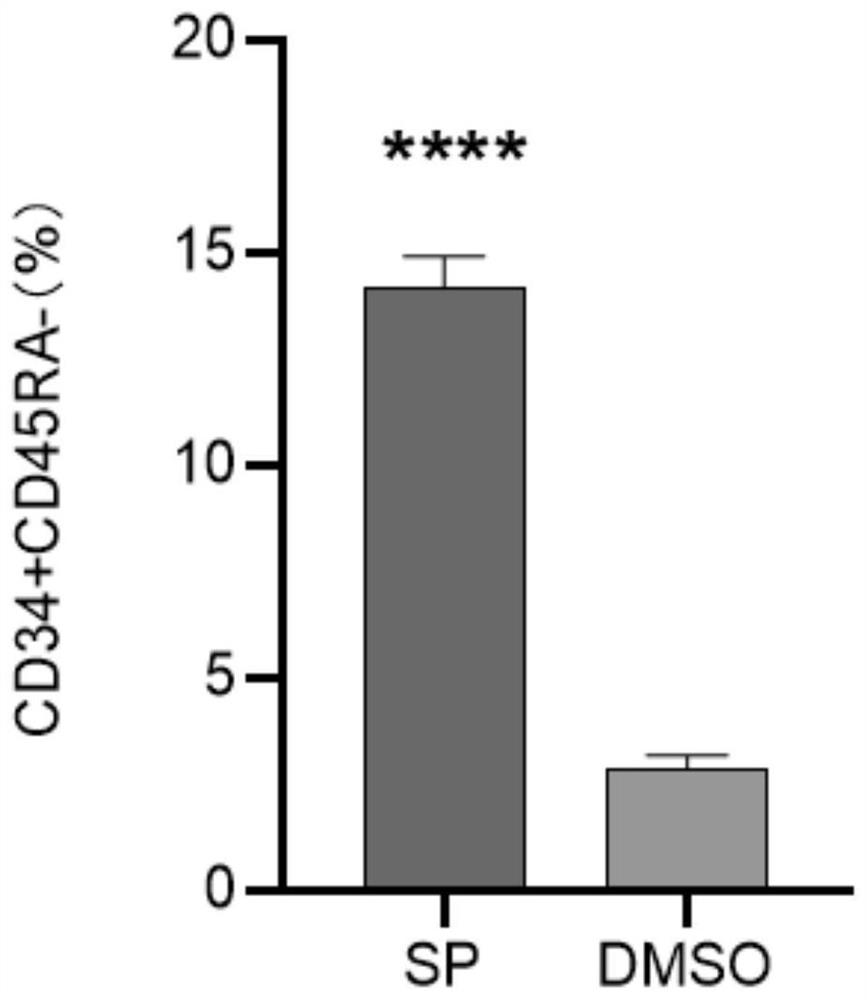
[0065] The result is as Figure 4 As shown, the results show that JNK inhibitors can significantly improve the colony-forming ability of hematopoietic stem cells.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com