A kind of preparation method of 2-ethoxy-5-fluorouracil impurity
A technology of fluorouracil and ethoxy, which is applied in the field of drug synthesis, can solve the problems of no public information reports, etc., and achieve the effect of short route and simple post-processing
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
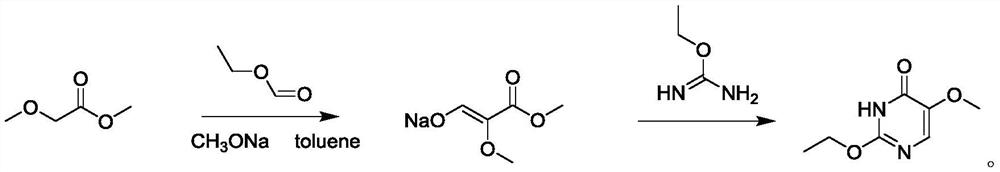
[0027] One aspect of the embodiment of the present invention provides a kind of preparation method of 2-ethoxy-5-fluorouracil impurity, it comprises the steps:
[0028] (1) take methyl methoxyacetate or ethyl ethoxyacetate as raw material, sodium methoxide is used as alkali extraction hydrogen, and toluene is used as the uniform mixing system of solvent to drip raw material and ethyl formate respectively, and react to form 2 -Ethoxy-5-fluorouracil impurity intermediate;
[0029] (2) Add ethoxyisourea dropwise to the 2-ethoxy-5-fluorouracil impurity intermediate, adjust pH=9-10, and then sequentially perform heating, vacuum distillation, water dissolving, and purification to obtain 2 -Ethoxy-5-fluorouracil impurity crude product;
[0030] (3) adding water to the 2-ethoxy-5-fluorouracil impurity crude product to dissolve, and then successively cooling and crystallizing with ice water, suction filtration, washing with water, and drying to obtain 2-ethoxy-5-fluorouracil impurity....
Embodiment 1
[0055] Synthesis of Impurity 1:
[0056] In a dry and clean 500mL three-necked flask, add 157g of methanol solution of sodium methoxide and stir, distill under reduced pressure to a fine powder, add 67g of solvent toluene, stir while adding, and control the temperature to 10-15°C, add 56g of ethyl formate and 56g of ethyl formate dropwise respectively. 35 g of methyl methoxyacetate was naturally raised to room temperature after the dropwise addition, heated to 40° C., incubated for 4-5 hours, returned to room temperature and left to stand overnight.
[0057] The temperature of the reaction system was controlled to 10-15°C, 95 g of ethoxyisourea was added dropwise, the pH was adjusted to 9-10, the temperature was heated to 40°C, and the reaction was maintained for 4-5h. The solvent was distilled off under reduced pressure, and the system was in the form of a dark yellow viscous sugar syrup. Add 100 g of water and stir to dissolve, add 30 ml of saturated brine to break the demul...
Embodiment 2
[0062] Synthesis of Impurity 1:
[0063] In a dry and clean 500mL three-necked flask, add 157g of methanol solution of sodium methoxide and stir, distill under reduced pressure to a fine powder, add 67g of solvent toluene, stir while adding, control the temperature to 10-15℃, and add 56.2g of ethyl formate dropwise. and 35.4 g of methyl methoxyacetate. After the dropwise addition, the mixture was naturally raised to room temperature, heated to 40° C., incubated for 4-5 hours, returned to room temperature, and left to stand overnight.
[0064] The temperature of the reaction system was controlled to 10-15°C, 99 g of ethoxyisourea was added dropwise, the pH was adjusted to 9-10, the temperature was heated to 40°C, and the reaction was maintained for 4-5h. The solvent was distilled off under reduced pressure, and the system was in the form of a dark yellow viscous sugar syrup. Add 100 g of water and stir to dissolve, add 30 ml of saturated brine to break the demulsification, extr...
PUM
| Property | Measurement | Unit |
|---|---|---|
| density | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
| flash point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com